Johns Hopkins APL Technical Digest
Large-Scale Production of Radiopure 135Xe from Bremsstrahlung γ-Irradiation of Solid Xenon Difluoride

Evan P. Lloyd, Ciara B. Sivels, Alan W. Hunt, and Yenuel S. Jones-Alberty
Abstract
The United States, together with the United Kingdom, signed the Limited Nuclear Test Ban Treaty in 1963. The Comprehensive Nuclear-Test-Ban Treaty was partially ratified by the United Nations General Assembly in 1996. A multifaceted worldwide monitoring network, in which the United States actively participates, continuously monitors treaty compliance. One of the tools this worldwide network uses is atmospheric sampling of radioxenon. During an underground detonation, noble gases, such as xenon, do not react with soil and can escape into the atmosphere. The detection of radioactive xenon in 2006 provided reliable proof of North Korea’s underground testing. Because radioactive xenon is required for calibrating the detectors, the synthesis of high-purity radioxenon is of interest. In light of this interest, a team at the Johns Hopkins University Applied Physics Laboratory (APL) developed a novel production pathway for the 135Xe isotope using APL’s new linear accelerator facility.
Download this article or scroll to continue reading
Introduction
This article describes the use of bremsstrahlung radiation to induce136 Xe(γ,p)135I reactions, followed by milking of 135Xe from β-decay of the 135I product. To increase Xe density in irradiated samples, we used solid xenon difluoride, resulting in a yield of 80 nCi 135I and 26 mCi 135Xe from 122 mg XeF2 after irradiation of 21.9 MeV over 30 min. Using XeF2, we were able to separate unreacted xenon from the 135I product via aqueous sodium hydroxide decomposition of XeF2, followed by removal of evolved gaseous xenon by cryotrapping with liquid nitrogen. After decay, radiopure 135Xe was recovered by cryotrapping.
Xenon-135, or 135Xe, is required to calibrate the detectors that passively monitor the atmosphere for global enforcement of the 1963 Nuclear-Test-Ban Treaty. Radioactive xenon in the atmosphere can indicate nuclear explosions or testing; it is a clear marker of underground testing (because the unreactive noble gas escapes and persists after a test). Indeed, monitoring 133Xe and 135Xe ratios offered the most reliable proof of North Korea’s 2006 underground nuclear test, providing a fission source term in contrast with seismic data that could have been produced with nonnuclear explosives.1,2 Optimized purification of xenon from atmospheric sampling has been demonstrated using the automated radioxenon sampler-analyzer (ARSA) since 1999. ARSA was developed by the US Department of Energy’s Office of Nonproliferation and the Pacific Northwest National Laboratory. Engineers have successfully purified and concentrated xenon from the atmosphere for analysis of radioxenon used to detect nuclear activity.
In experimental nuclear physics, a two-body nuclear reaction can be expressed as A(a,b)B, where, by convention, A represents the target nucleus; a is the incident particle; b is a lighter ejected particle, known as the ejectile; and B is a heavier ejected particle, known as the recoil. 134Xe(n,γ)135Xe transitions after exposure of low-density gaseous xenon, in which a neutron is incident on a 134Xe nucleus, produce valuable 135Xe samples for calibrating the worldwide detectors. These samples, however, contain mostly 134Xe, which complicates the calibration process. Not only would radiopure and carrier-free 135Xe samples improve the existing state of the art, but using an alternative, denser form of Xe would allow for production of greater yields.
136Xe(γ,p)135I reactions have been described as a way to produce 135Xe. Horkley et al. recently reported production of 592 Bq of 135Xe after 6.5-h irradiation at 21.8 MeV.3 These experiments produced 135Xe without detectable 133Xe; however, the yields were 103 times lower than would be required for practical use. If using a similar gamma source, concentrating the xenon substrate solves this limitation.
Xenon difluoride, or XeF2, is a colorless salt produced from the reaction of xenon and difluorine. These gases react when energy is added. XeF2 has been synthesized from irradiation with ultraviolet (UV) light, heat, γ-rays, and other energy sources.4 Today XeF2 is typically produced via UV irradiation of a xenon fluorine mixture in a 2:1 ratio with catalytic 1 mol % hydrogen fluoride,5 which forms pure crystals. Because of its volatility and reactivity, XeF2 is best stored cold and in a sealed polyethylene or fluoropolymer container. Although xenon is a noble gas and therefore tends to be chemically inert, xenon difluoride is reactive; it will react with water but not rapidly, and it requires the presence of base to reliably dissociate and evolve xenon gas.6 XeF2 is used as a niche fluorinating agent for research purposes7 but has limited industrial use synthetically. XeF2 is available commercially from common chemical suppliers and can be purchased for less than $200 per gram, although not in isotopically pure forms. Furthermore, purifying xenon from XeF2 is simple by using liquid nitrogen for cryo-condensation.
Efforts to optimize the purification of xenon from atmospheric sampling have demonstrated reliable sequestration of radioxenon produced from 136Xe(γ,p)135I reactions and subsequent 135I β-decay. In this article, we report a proof of concept for generating 135Xe in large yields from a solid xenon-containing substrate, followed by purification and trapping of radiopure 135Xe.
Linear Accelerator Facility
We conducted experiments in APL’s linear accelerator facility, a ~4,000-ft2 laboratory that consists of a ~1,000-ft2 heavily shielded accelerator hall, a ~600-ft2 heavily shielded experimental hall, a ~620-ft2 radiochemistry laboratory, and a ~520-ft2 experiment staging area. The accelerator and experimental halls are both partially underground with walls and ceilings consisting of ~3 ft of concrete and 1.7 ft of steel, providing ample radiation shielding for radiation-producing machines and large radioactive sources. The accelerator is capable of delivering electron pulses with operator-selectable widths between 0.5 and 4 ms at a repetition rate up to 115 Hz. The maximum charge per pulse ranges from 40 nC for 0.5-ms pulses to 350 nC for 4-ms pulses. The electron energy can be varied from ~5 to 25 MeV with an energy resolution of 5%, which can be reduced using collimators or slits. The S-band standing wave radio frequency linear accelerator, which operates at ~2.8 GHz, was used to accelerate 21.9-MeV electrons that generate bremsstrahlung γ-radiation using a 2-mm tungsten source. With the electron linear accelerator’s energy range and versatility, we were able to develop a novel solution for synthesizing radiopure 135Xe.
Experimental Methods
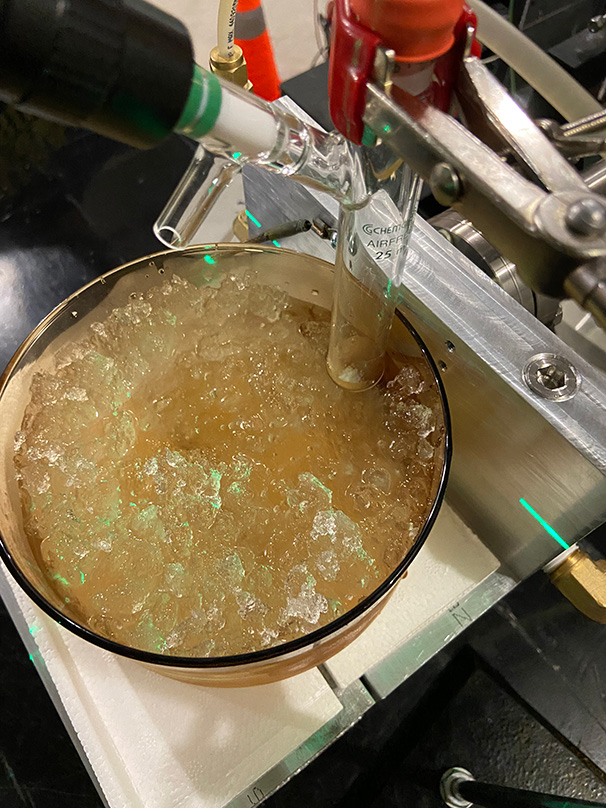
We placed xenon difluoride crystals (200 mg) into a 25-mL single-neck borosilicate glass Schlenk reaction tube fitted with a rubber septum. Next we submerged the sealed reaction tube in an ice water bath and then placed it directly in the γ-beam. This setup is pictured in Figure 1.
Figure 1
Placement of the reaction tube containing XeF2 crystals in APL’s linear accelerator facility γ-beam.
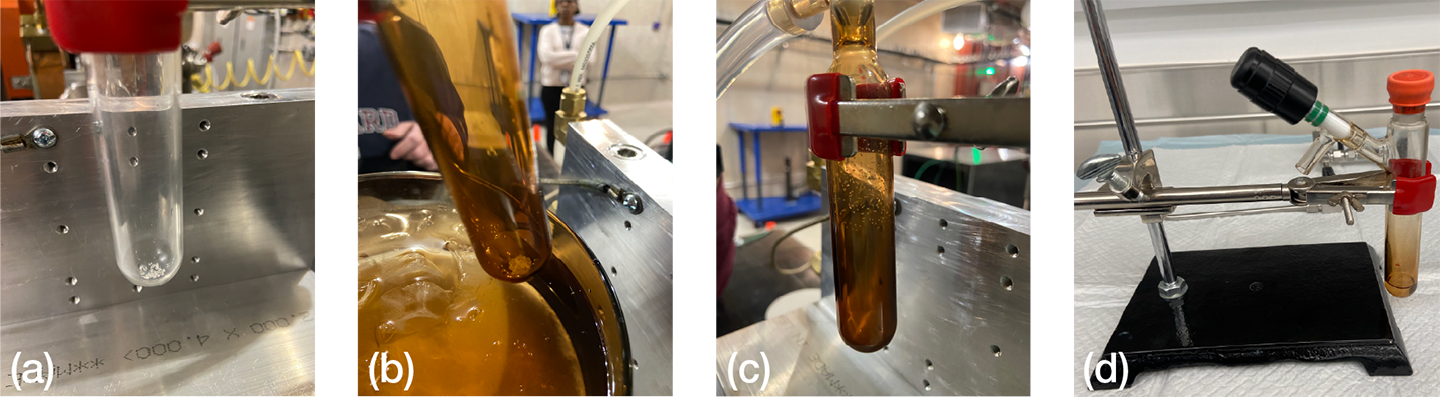
We fit the sealed reaction tube containing irradiated XeF2 to the apparatus pictured in Figure 2. The apparatus comprised the sealed reaction tube connected to a drying trap that facilitated visualization of moisture trapping. The drying trap connected to a stainless steel U-tube, also shown in Figure 2, and was fitted with a manual valve on both ends and a gauge to monitor internal pressure. We introduced into the reaction tube a steady stream of ultrahigh-purity nitrogen from a cylinder. Next, we opened the tube, drying tube, and reaction tube valves and then submerged the U-tube in liquid nitrogen before adding aqueous sodium hydroxide (1 N, 3 mL) to the XeF2 crystals. Nitrogen flowed into the reaction solution to sparge the mixture and remove the entirety of dissolved xenon, as shown in Figure 3. We continued to purge the system with nitrogen for 1 min after we could no longer see xenon evolution, and then we shut off the gaseous nitrogen flow. Finally, we closed the reaction tube, drying tube, and tube valves and removed the U-tube from the liquid nitrogen bath, allowing the tube to warm to room temperature. Figure 4 shows the XeF2-containing reaction tube at various stages during these experiments.
Figure 2
The apparatus used to purify xenon from XeF2 Inset, the U-tube fitted with a pressure gauge and valves on either end containing cryotrapped xenon.

Figure 3
Removal of dissolved xenon gas by sparging XeF2 dissolved in 1N NaOH with gaseous nitrogen.

Figure 4
The XeF2-containing reaction tube. (a) Crystals before irradiation. (b) Intact crystals after irradiation at 0°C. (c) Sublimation of crystals after irradiation at ambient temperature. (d) Solution after dissociation with 1N NaOH.
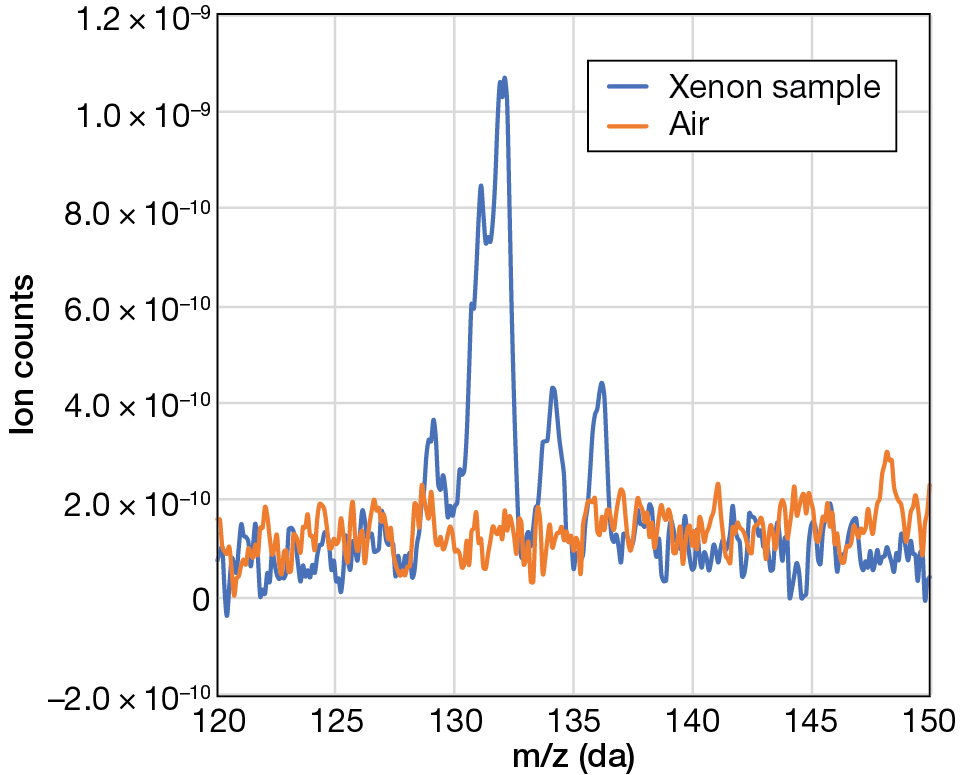
We piloted this procedure with unirradiated XeF2 and examined the collected xenon by residual gas analysis, which is a form of mass spectrometry. During mass spectrometry, the mass-to-charge ratio (m/z) of an ion is measured, typically by manipulating the ion’s trajectory using a magnetic field.8 The sensitivity of mass spectrometry techniques is such that individual ions of a given mass-to-charge ratio can be counted. This analysis revealed xenon isotopes ranging from 126 to 136 Da, similar to the natural isotopic distribution for xenon, as shown in Figure 5. An increase in ion counts can be observed in this range for the xenon sample, shown in blue.
Figure 5
Residual gas analysis of cryotrapped xenon from XeF2.
Results
Irradiation of Xenon Difluoride
We sought to irradiate xenon difluoride crystals and determine the yield of 135I and 135Xe produced from a solid substrate. In a first-pass experiment, we added 100 mg XeF2 to a borosilicate Schlenk tube. Processes to synthesize XeF2 involve adding energy during the reaction, including γ-irradiation, and we did not know whether adding high amounts of ionizing radiation could reverse the reaction and produce hazardous gases.9 Therefore, we fit the Schlenk tube to a gas scrubber to remove any produced hydrogen fluoride or fluorine vapors with aqueous 3N sodium hydroxide. This precaution turned out to be unnecessary, however, as we observed no bubbling during irradiation, and the XeF2 crystals appeared intact after irradiation for 15 min at 21.9 MeV. Longer irradiations, at 1 h, resulted in visible sublimation of the XeF2 substrate, so we performed future experiments at 0°C by using an ice water bath. With the temperature held constant at 0°C, we observed no sublimation, and the XeF2 crystals remained intact and in place at the bottom of the reaction tube.
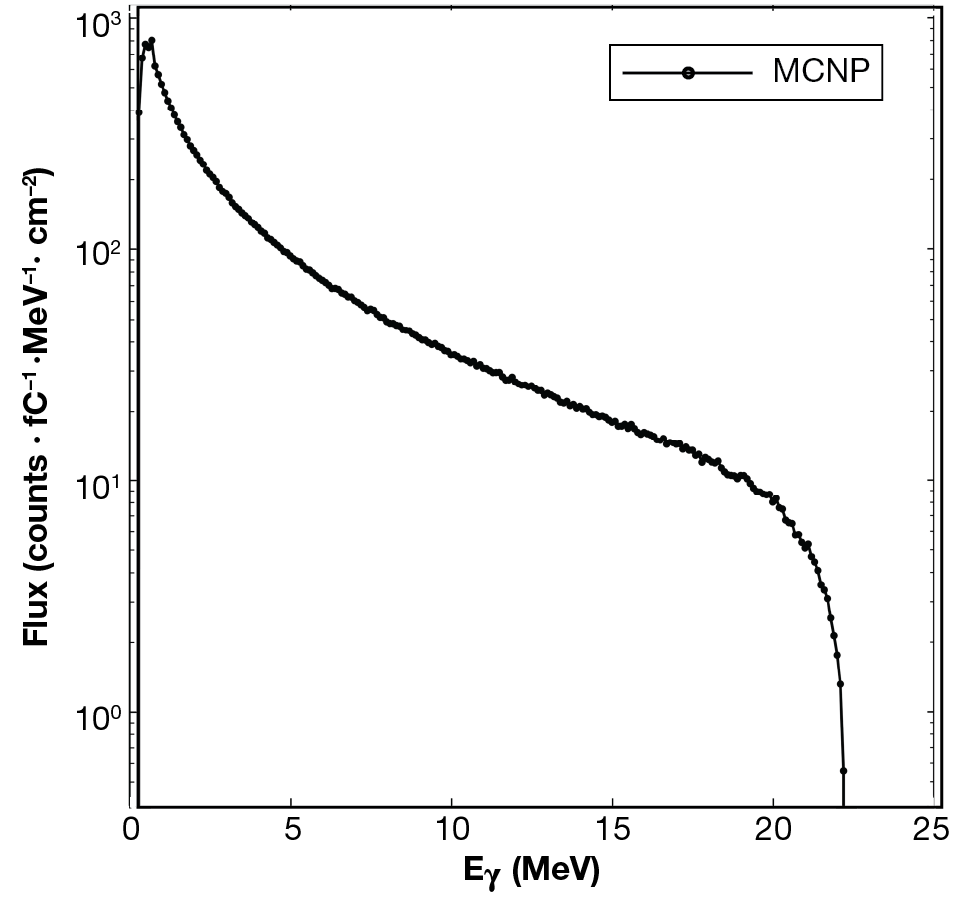
We measured the production yields of 135I and 135Xe at multiple energies by bombarding a 200-mg sample of XeF2 with bremsstrahlung radiation. Measurements were taken at 16-, 19-, and 22-MeV end-point energies at 15-min irradiation intervals. Electrons were accelerated using the linear accelerator facility’s 25-MeV linear accelerator (Figure 6), and bremsstrahlung radiation was generated by impinging a 2-mm-thick tungsten radiator. Also known as braking radiation, bremsstrahlung results from electrons decelerating in a medium.11,12 A 2.54-cm-thick block of aluminum served as a beamstop. A simulated bremsstrahlung spectrum for an incident 22-MeV electron beam is shown in Figure 7.
Figure 6
The 25-MeV linear accelerator in APL’s facility.
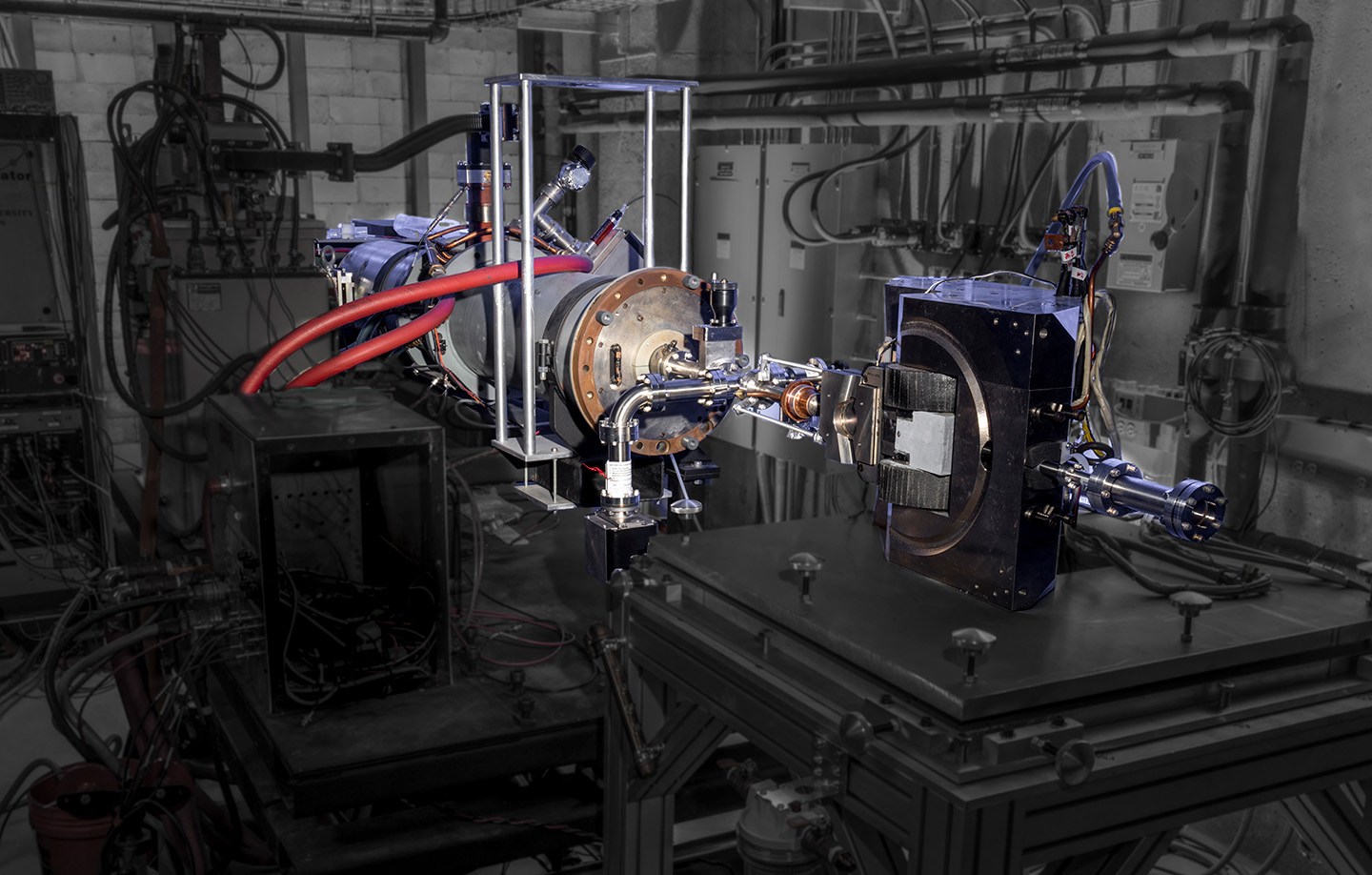
Figure 7
Monte Carlo N-Particle (MCNP)10 simulated bremsstrahlung spectrum for an incident 22-MeV electron beam.
Purification of Xenon from Xenon Difluoride
We hypothesized that XeF2 could be rapidly dissociated into xenon gas and fluoride salts upon addition of aqueous sodium hydroxide. Indeed, adding 3 mL 1N NaOH solution to the XeF2 crystals caused vigorous bubbling in our initial tests with unirradiated material. We assembled an apparatus to remove xenon after irradiation by using positive flow of nitrogen gas from a cylinder into the sample-containing Schlenk tube, followed by drying of the flowed gases through anhydrous calcium sulfate desiccant and condensation of xenon gas into a stainless steel trap made from ¼-in. stainless steel tubing using liquid nitrogen. Xenon was cryotrapped from unirradiated XeF2 using our apparatus, and successful xenon recovery was validated by residual gas analysis mass spectrometry.
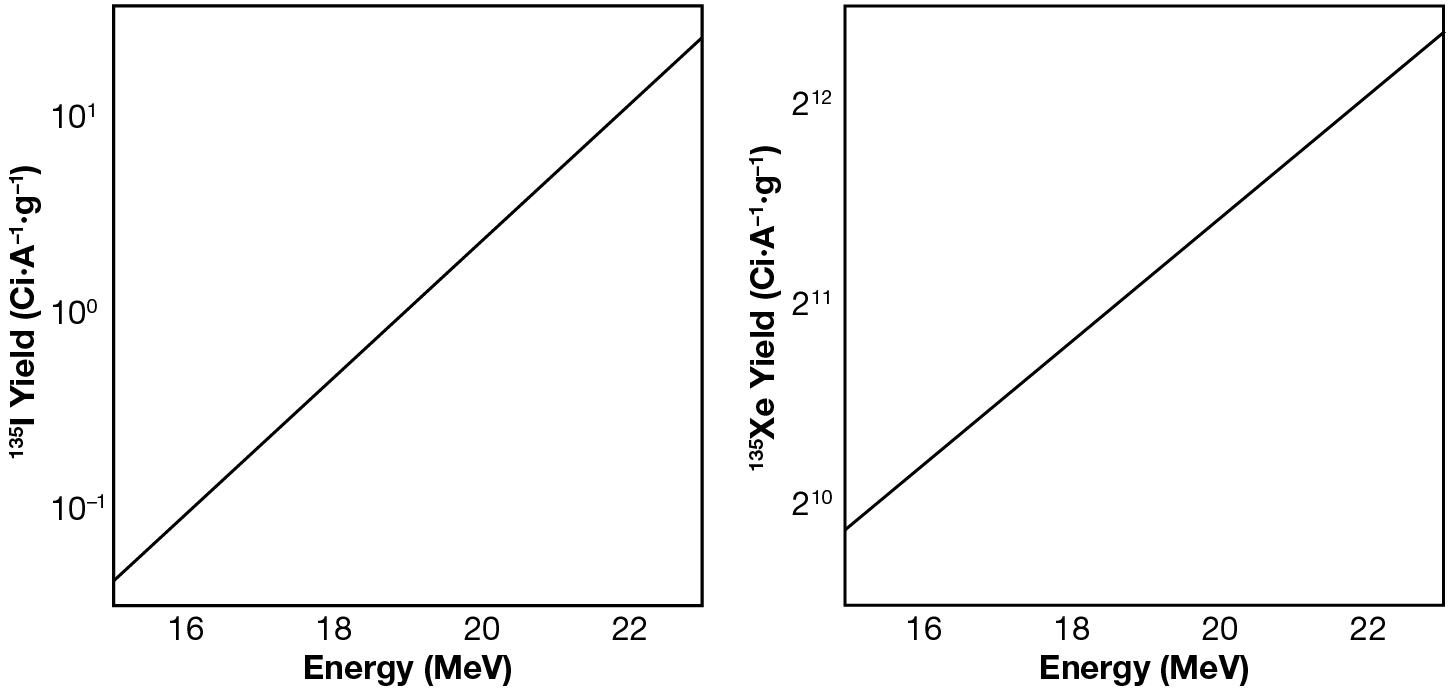
The experimental workflow was repeated with irradiated XeF2 and iodine was successfully maintained in the aqueous sodium hydroxide solution. The isotopes of interest, 135I and 135Xe, have half-lives of 6.9 ± 0.4 h13 and 9.13 ± 0.5 h,14 respectively. Furthermore, it can be extrapolated that 135I β-decays exclusively to 135Xe based on the energies reported for this transition.15 Characteristic γ-decay energies associated with these radioisotopes were measured by analyzing the cryotrapped gases using a high-purity germanium detector (HPGe), which is a semiconductor γ-ray detector.11 We observed prominent γ-ray decays of energy Eγ = 1,260.409, 1,131.511, and 1,457.56 keV, as shown in Figure 8. Decay curves, shown in Figure 9, were constrained for each isotope and are within error bars of known values. The production yields for 135I and 135Xe are shown in Figure 10. We determined that 135Xe was successfully purified from the 135I sample. After waiting for ~16 h, we repeated the procedure to sparge the solution and induce cryotrapping of the 135Xe resulting from β-decay of the 135I product during that period. Radiopure 135Xe was recovered.
An additional insight resulted from these measurements. Currently, the experimental cross sections of the 136Xe(γ,n)135Xe and 136Xe(γ,p)135I reactions, which describe the probability of these reactions taking place, have not been measured. As such, theoretical cross sections are used to predict the production yields of the 135I and 135Xe isotopes. Our measured 135I production yield suggests a large discrepancy between the theoretical and experimental cross sections. Further measurements are needed to clarify this discrepancy and to constrain the experimental cross sections for these reactions; this work would be beneficial to the nuclear data community.
Figure 8
HPGe gamma spectrum for post-irradiated XeF2.
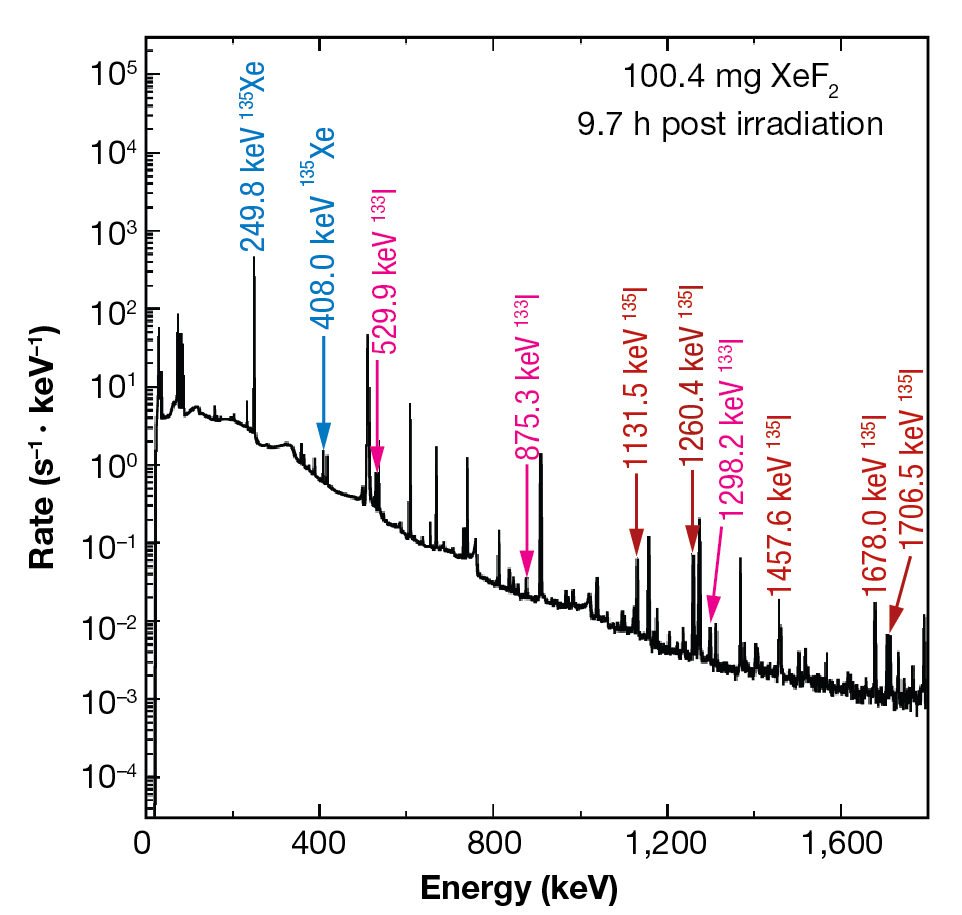
Figure 9
Decay curves for 135I and 135Xe produced from irradiation of XeF2.
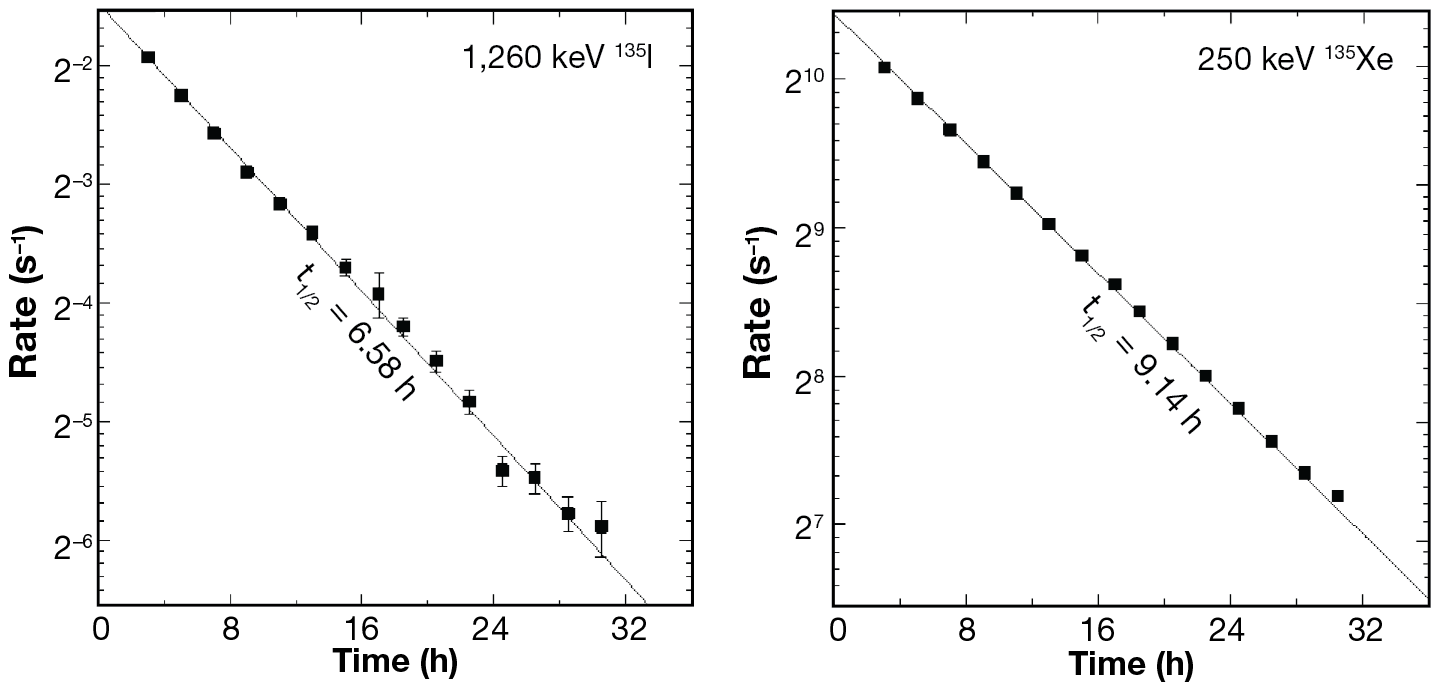
Figure 10
Production yields of 135I and 135Xe at 16, 19, and 22 MeV.
Conclusion and Outlook
Our hypothesis for how 135Xe could be purified from the irradiated solid XeF2 hinges on the passive evolution of gaseous xenon from aqueous solution. The overall radiochemical transition from 136Xe to 135I is shown in Figure 11. As the 135I is likely stable as a salt in aqueous solution, the remaining xenon can be removed at this point by sparging the aqueous solution with an inert gas, such as nitrogen. The remaining aqueous 135I will decay into 135Xe with a half-life of 6.6 h and then can be removed by sparging the solution with gaseous nitrogen. Xenon’s boiling point is 165 K, compared with nitrogen’s boiling point of 77.3 K; therefore, the evolved 135Xe can be trapped using liquid nitrogen.
Figure 11
Radiochemical irradiation of XeF2 and extraction chemistry.
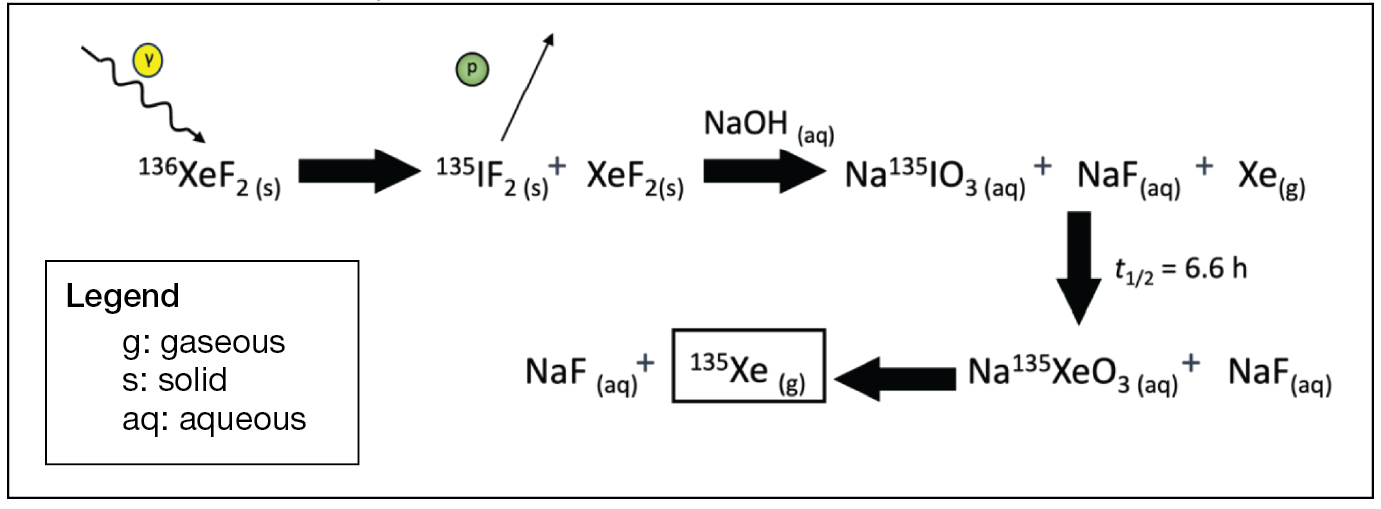
The proposed chemistry in Figure 11 allows 135Xe to be selectively purified if radiopure 136XeF2 is used because the unconverted xenon is removed after the addition of aqueous sodium hydroxide (NaOH) followed by sparging of the solution with nitrogen. The removal of evolved 135Xe after a waiting period through cryotrapping leaves behind the 135I as an aqueous salt. The purity of the milked 135Xe is measured using HPGe γ-spectroscopy, demonstrating the effectiveness of this approach for large-scale 135Xe production. An example of the HPGe γ counting setup is shown in Figure 12.
Figure 12
An example of the HPGe γ counting setup. The HPGe, shown on the right side of the image, is housed in lead bricks.
These methods could be applied to a more refined production process using a custom-built and engineered apparatus. The techniques involved are simple and rely on separation of undesired xenon isotopes by removing them before the majority of the 135I decay. If inert conditions are maintained through the use of a nitrogen atmosphere, using liquid nitrogen to cryotrap the radioactive xenon product gas is a reliable approach to separating 135Xe from the mixture without risking chemical release as a result of system overpressure.
Using XeF2 as a substrate for radiochemical production of 135Xe is a viable strategy to generate calibration samples for the comprehensive Nuclear Test Ban Treaty’s atmospheric xenon detectors. Not only are production yields sufficient, but the ability to chemically separate the produced 135I allows for isolation of radiopure 135Xe and removes the presence of problematic xenon isotopes. Cryo-condensation of these undesirable xenon isotopes allows for their safe handling and recycling, as well as bottling of radiopure 135Xe for future sample preparation.
Future directions to refine this approach would involve designing a 135Xe separation apparatus and using radiopure 136XeF2. An apparatus for 135Xe evolution and separation would require the use of an inert atmospheric environment, as previously stated, so the ability to load the irradiated XeF2 material and purge the system with gaseous nitrogen is required, followed by the ability to add aqueous sodium hydroxide solution to the XeF2 and sparge the resulting solution without opening the system to the atmosphere. Ideally, the evolved Xe that contains undesired 136Xe from the sample would be cryotrapped and recycled into the synthesis of 136XeF2. The apparatus should allow for an additional or replacement cryotrap vessel so that pure 136Xe can be collected after the 135I decay process without opening the system to the atmosphere.
Radiopure 136XeF2 can be synthesized through exposing UV light to a mixture of radiopure gaseous 136Xe, available from chemical vendors in high purity, and fluorine gas, which is also commercially available. Although gaseous fluorine is highly corrosive and toxic, other researchers have described the synthesis of XeF2 in a controlled laboratory setting.4 APL researchers could reproduce these settings in their laboratory or contract chemical manufacturers to do so (though the latter option would be more expensive).
We were able to demonstrate a novel production pathway for the synthesis of radiopure 135Xe through three innovations: the milking of 135Xe via the 136Xe(γ,p)135I reaction (where a XeF2 target was bombarded with bremsstrahlung radiation generated by the 25-MV linear accelerator), the use of a solid XeF2 target instead of a liquid solution (which increases the density of Xe in the target), and the chemical extraction of 135I from the irradiated XeF2 target. If implemented, our production pathway for radiopure 135Xe could produce sufficient radioxenon to supply calibration material to the entirety of the worldwide network of detectors that passively sample radioxenon from the atmosphere.
1. P. R. J. Saey, M. Bean, A. Becker, J. Coyne, R. d’Amours, et al., “A long distance measurement of radioxenon in Yellowknife, Canada, in late October 2006,” Geophys. Res. Lett., vol. 34, no. 20, art. L20802, 2007, https://doi.org/10.1029/2007GL030611.
2. R. L. Garwin and F. N. von Hippel, “A technical analysis: Deconstructing North Korea’s October 9 nuclear test,” Arms Control Today, vol. 36, no. 9, pp. 14–16, Nov. 2006, https://www.armscontrol.org/act/2007-03/features/technical-analysis-deconstructing-north-korea’s-october-9-nuclear-test.
3. J. J. Horkley, J. L. Brookhart, E. S. Cardenas, K. P. Carney, C. Hines, T. P. Houghton, T. A. Robinson, and M. G. Watrous, “Production of xenon-135 from isotopically enriched xenon-134 and xenon-136 targets,” J. Radioanalyt. Nucl. Chem., vol. 331, pp. 5447–5452, 2022, https://doi.org/10.1007/s10967-022-08537-1.
4. M. Tramšek and B. Žemva, “Synthesis, properties and chemistry of xenon(II) fluoride,” Acta Chim. Slov., vol. 53, no. 21, pp. 105–116, 2006, https://doi.org/10.1002/chin.200721209.
5. A. Šmalc, K. Lutar, and S. A. Kinkead, “Xenon difluoride (modification),” in Inorganic Syntheses, vol. 29, R. N. Grimes, Ed., New York: Wiley, 1992, pp. 1–4, https://doi.org/10.1002/9780470132609.ch1.
6. E. H. Appelman, “Reaction of xenon difluoride with water and with xenon trioxide,” Inorg. Chem., vol. 6, no. 7, pp. 1305–1310, 1967, https://doi.org/10.1021/ic50053a009.
7. C. A. Ramsden, “Xenon difluoride in the organic laboratory: A tale of substrates, solvents and vessels,” ARKIVOC, vol. 2014, no. 1, pp. 109–126, 2014, https://doi.org/10.3998/ark.5550190.p008.436.
8. G. Glish and R. Vachet, “The basics of mass spectrometry in the twenty-first century,” Nat. Rev. Drug Discov., vol. 2, pp. 140–150, 2003, https://doi.org/10.1038/nrd1011.
9. D. R. MacKenzie and R. H. Wiswall Jr., “Compound formation by γ-irradiation of xenon-fluorine mixtures,” Inorg. Chem., vol. 2, no. 5, p. 1064, 1963, https://doi.org/10.1021/ic50009a044.
10. M. E. Rising, J. C. Armstrong, S. R. Bolding, F. B. Brown, J. S. Bull, et al., “MCNP® code version 6.3.0 release notes,” Los Alamos Nat. Lab. Tech. Rep. LA-UR-22-33103, Rev. 1, Jan. 2023.
11. J. E. Turner, Atoms, Radiation, and Radiation Protection. Wiley-VCH, Germany, 2007.
12. G. F. Knoll, Radiation Detection and Measurement. 4th Ed. Wiley, 2010.
13. Y. S. Popov, L. V. Zakharova, and G. A. Timofeev, “Half-lives of 131–135I nuclides,” Radiochemistry, vol. 43, pp. 5–7. 2001, https://doi.org/10.1023/A:1012805618385.
14. F. Brown and L. Yaffe, “The independent yield of Xe135 produced in the fission of natural uranium by pile neutrons,” Can. J. Chem., vol. 31, pp. 242–249, 1953, https://doi.org/10.1139/v53-035.
15. B. Singh, A. A. Rodionov, and Y. L. Khazov, “Nuclear data sheets for A = 135,” Nucl. Data Sheets, vol. 109, no. 3, pp. 517–698, 2008, https://doi.org/10.1016/j.nds.2008.02.001.
Evan P. Lloyd is an organic chemist in APL’s Asymmetric Operations Sector. He has a B.S. in chemistry from Carnegie Mellon University and an M.S. and a Ph.D. in chemistry from Johns Hopkins University. He has experience in antibiotic drug development, medicinal chemistry, total synthesis, and enzymology. At APL, he has worked in the chemical and biological warfare agent defeat community, specializing in applied synthetic chemistry and testing.
Ciara B. Sivels is a nuclear engineer in APL’s Air and Missile Defense Sector. She has a B.S. in nuclear engineering from the Massachusetts Institute of Technology (MIT) and an M.S. and a Ph.D. in nuclear engineering from the University of Michigan. She has experience in radiation detection and simulation, nuclear explosion monitoring for treaty verification using scintillation detectors, Monte Carlo radiation transport simulations, and high-resolution low-background measurements using semiconductor detectors.
Alan W. Hunt supervises the Radiation Systems Technologies Section within APL’s Asymmetric Operations Sector. He has a B.S. in physics from the University of Michigan and a Ph.D. in physics from Harvard University. He led the effort to establish APL’s linear accelerator and high-radiation facility. His research projects have spanned liquid semiconductors for novel power generation applications to electron/ion-induced spaceship charging. His most prolific research area has been investigating and developing nuclear techniques for security, nonproliferation, and nuclear forensics applications. The common theme in all his research endeavors is the interaction of ionizing radiation with matter and how to exploit the associated phenomenon for applications.
Yenuel S. Jones-Alberty is a postdoctoral fellow in APL’s Asymmetric Operations Sector. He has a B.S. in physics from the University of Puerto Rico and an M.S. and a Ph.D. in physics from Ohio University. He has worked on various projects in APL’s linear accelerator facility, ranging from specific applications to basic science, and has operated the linear accelerator. His graduate research focused on studying nuclear reactions of astrophysical significance using neutron detection techniques. He operated a 4.50-MV tandem Pelletron accelerator when performing these experiments.