Press Release
Breakthrough Cuttable, Flexible, Submersible and Ballistic-Tested Lithium-Ion Battery Offers New Paradigm of Safety and Performance
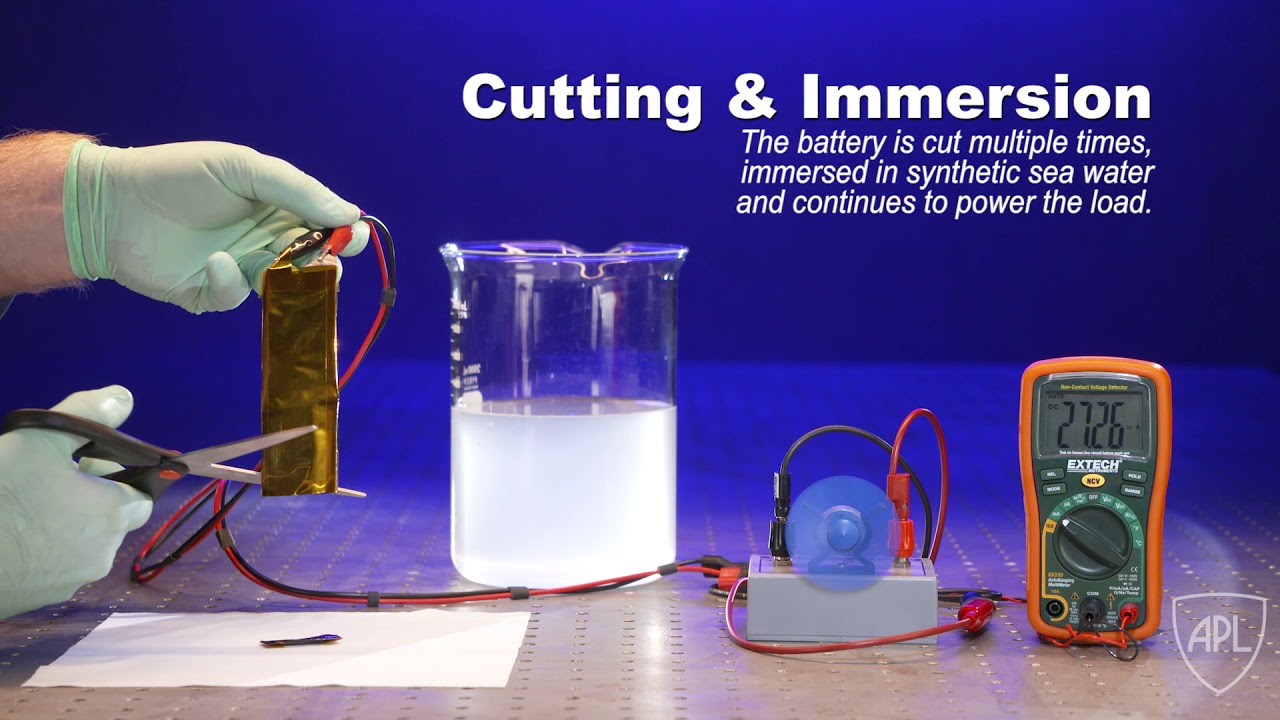
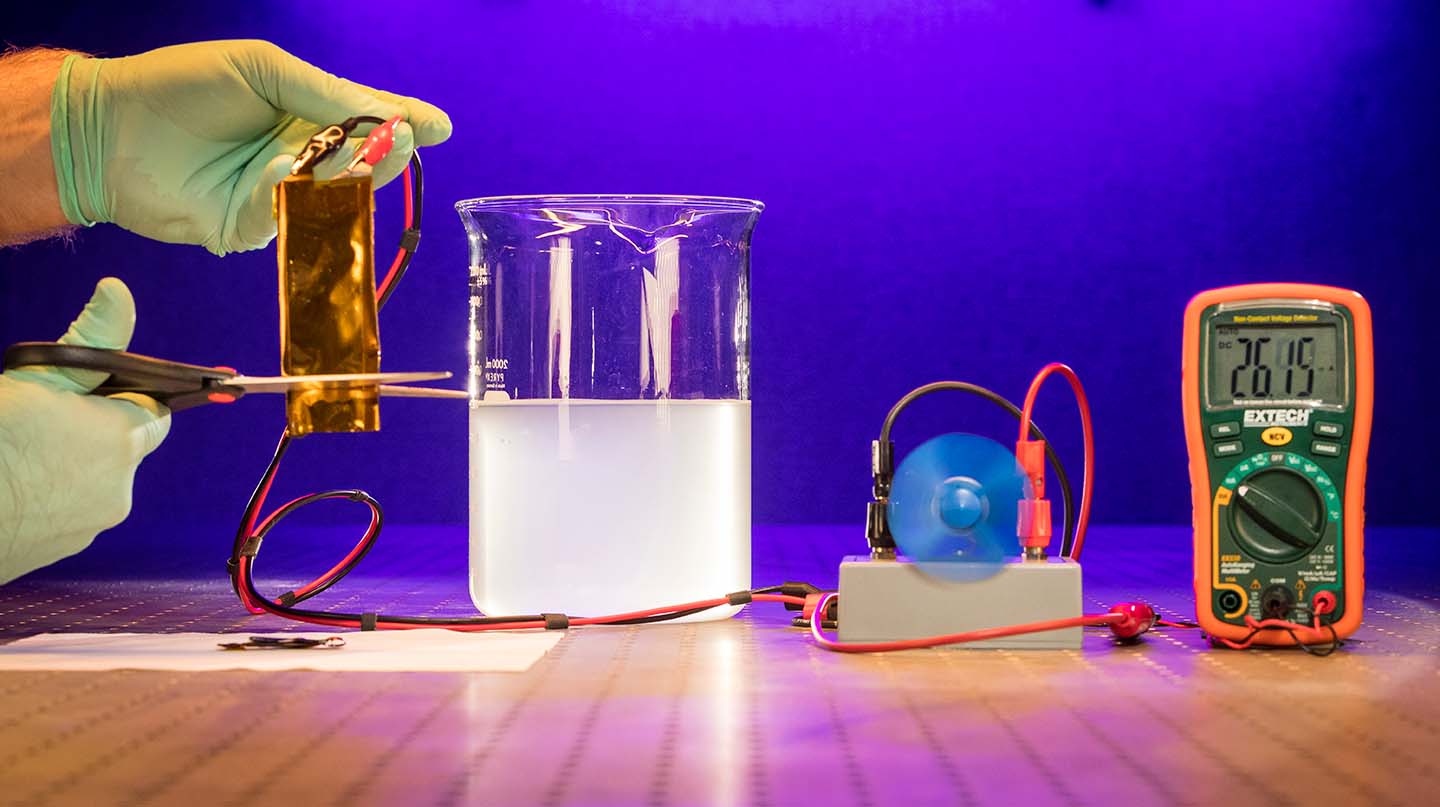
A team of scientists at the Johns Hopkins University Applied Physics Laboratory (APL) in Laurel, Maryland, has partnered with researchers from the University of Maryland (UMD) and the Army Research Laboratory (ARL) to develop a new type of flexible lithium-ion battery that can operate under extreme conditions, including cutting, submersion and ballistic impact. The team recently published their discovery in the journal Advanced Materials.
Li-ion batteries have become the energy storage source of choice for multiple applications, ranging from consumer electronics to military and aerospace systems, due to their energy and power performance. Despite these benefits, potential safety hazards associated with the organic electrolytes that are used in Li-ion battery cells remain an ongoing concern. These electrolytes are highly flammable, toxic, and moisture sensitive, limiting the forms in which a Li-ion battery can be manufactured.
In the recently published paper “Flexible Aqueous Li-ion Battery with High Energy and Power Densities” in Advanced Materials, a team of scientists from UMD, APL, and ARL have demonstrated a new type of flexible Li-ion battery that is not hazardous and also can continue to operate even under severe mechanical abuse.
The work builds upon a novel aqueous electrolyte referred to as “water-in-salt” developed in 2015 by UMD and ARL. This highly concentrated water-based electrolyte can address the key issue associated with the use of water in Li-ion batteries, which is the low electrochemical stability window of roughly 1.2 volts. By expanding this window to 3 volts, the water-in-salt enables much higher energy density aqueous Li-ion batteries.
“In recent years, UMD and ARL have explored several anode and cathode combinations that can be used within the stability window of our electrolyte. By collaborating with APL, we are starting to transition this technology into novel battery architectures and demonstrate its practical true potential,” said Chunsheng Wang, professor of chemical and biomolecular engineering at UMD and corresponding author of the paper.