Johns Hopkins APL Technical Digest
Simulants for Chemical and Explosive Threats

David S. Lawrence and Kelly A. Van Houten
Abstract
Studies using chemical warfare agents (CWAs) and explosives are dangerous and are therefore conducted only in specialized laboratories with highly controlled conditions and limited accessibility. Obtaining these materials for study is another challenge, as they are tightly regulated. Because of these challenges, simulants—molecules that mimic key characteristics of a specified CWA or explosive but lack toxicity—are often used in testing, sensor development, and decontamination studies. In the past, simulants have generally been selected on the basis of their historical use (with researchers sometimes simply choosing “convenient” materials that happen to provide a detector alarm, for example) rather than rational design. The Johns Hopkins University Applied Physics Laboratory (APL) created a simulant development approach, based on systems engineering concepts, that takes input from relevant parties and is scoped specifically for a project objective. This methodology can be universally applied to any type of simulant and includes down-selection criteria to identify specific simulant candidates that can be verified and validated according to the project’s required fidelity. This article describes the development approach, selection process, and demonstrated use cases for both CWAs and energetic materials.
Download this article or scroll to continue reading
Introduction
The use of chemical warfare agents (CWAs) is a growing threat. Between 1970 and 2020, more than 200,000 global terrorist attacks were reported in the Global Terrorism Database (GTD),1 but just over 400 involved a CWA. However, from 2013 to 2017, a 500% annual increase of CWA-related incidents (193 attacks) was reported.1 Recent events include the 2018 attack in Salisbury, United Kingdom, with a reported Novichok agent;2 the 2017 assassination of the half-brother of Kim Jong Un with the nerve agent VX;3 and over 1,400 deaths attributed to chemical warfare in the Syrian conflict.4,5
Adversarial use of energetic, or explosive, materials is also a continuing threat. For the calendar year 2022, the US Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) Bomb Arson Tracking System (BATS) reported 14,627 explosives-related incidents in the United States.6 Although the number of US incidents has varied slightly over the past couple of decades (since 9/11), it has been consistently high and reflects the much higher number of incidents occurring worldwide.
These trends highlight the need to study toxic CWAs and energetic materials and the consequences of their use, but work with these materials is potentially hazardous and usually fraught with challenges—such as government regulation in gaining access to specialized laboratories, licensing, and accessing materials (especially chemical agents on the Chemical Weapons Convention list of scheduled chemicals7). For these reasons, it has become necessary to develop simulants—materials that mimic the relevant properties of the “target” chemical agent or energetic material. For example, simulant materials can be used for testing detection capabilities, exercising decontamination protocols, or training. Each application will have specific target attributes that need to be simulated with a required degree of fidelity. While some simulants may be adequate for more than one application, each simulant is generally tailored for a particular use case (such as detection).
Since 2006, APL researchers have been preparing simulants and test articles for explosives, CWAs, and narcotics threats for the Department of Defense and the Department of Homeland Security (DHS). In efforts for DHS and the Transportation Security Administration (TSA), APL developed simulants for explosives and synthetic opioids. This article focuses on APL’s simulant-based efforts for CWAs and energetic threats; APL’s work on simulants for illicit drugs is outside the scope of this article but is an active area of research.
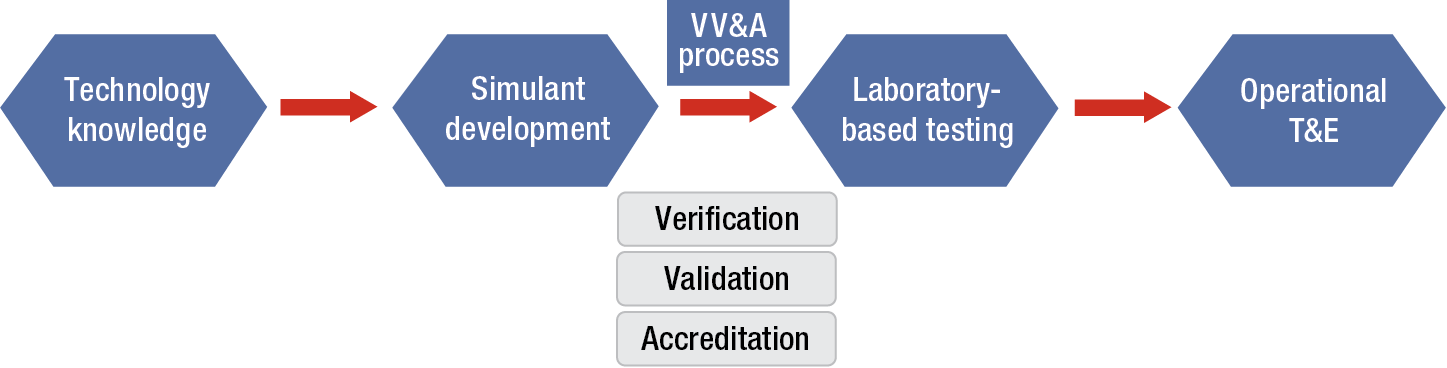
APL has established guidelines for a rigorous and successful design and testing program, shown schematically in Figure 1 for test and evaluation (T&E) applications. The overarching approach to simulant development is based on a “systems engineering” philosophy that begins with understanding the intended use for the simulant and how it contributes to the entire T&E process. The first step, “technology knowledge,” requires end-user input to help establish selection parameters and includes relevant target data so that the target–simulant characteristics used for the application can be correlated during the selection process.
Simulant development for both CWAs and energetic materials follows equivalent design and development paradigms. It is important that the entire process—particularly the operational use of the material—be kept in mind when designing simulant materials. Development of simulants for other applications, such as decontamination, follows similar approaches.
Figure 1
Standardized simulant development process for operational T&E. It is critical that the entire process, especially the operational use of the material, be considered during development.
Explosive Simulants
In 2007, APL developed a generic verification, validation, and accreditation (VV&A) methodology for use with any simulant and any detection technology. The VV&A methodology begins with a detailed understanding of the detection technology and algorithms of interest. Verification involves comparing relevant detection parameters for the energetic target and any candidate simulants. One key technology for detecting energetic materials is based on x-ray imaging. X-ray-based detection systems generally rely on material characteristics that include the effective atomic number and the density of the material (mass per unit volume). A related value, the CT (computed tomography) number, is also used in some x-ray screening systems as a way of describing the x-ray “texture” of a material. These characteristics can be calculated and/or measured for both energetic targets and simulant materials.
Validation of the candidate simulants involves side-by-side comparisons with the appropriate energetic target in the detection system under evaluation. For these purposes, the “system” includes not only the hardware but also the firmware and any software algorithms currently in place. A sufficient number of test runs with both the energetic target and the simulant must be performed using the system, in all relevant orientations of test samples (depending on the type of detection system), to ensure statistical significance of the probability of detection and probability of false alarm values within the desired level of confidence.
Accreditation involves the determination that the chosen simulant will perform appropriately under the conditions of its intended use. For an operational test, such as in an airport security checkpoint, the simulant will have to be usable in the local environment (e.g., in similar temperature and humidity conditions). It will also need to be nontoxic to humans and safe to use in indoor environments. The material’s shelf life should exceed the duration of the testing period, at a minimum. Finally, the material should be user-friendly for the intended application—for example, when contained in a piece of luggage with other background materials, such as clothing and electronics (“clutter”), the material should be packaged in such a manner that it can be manipulated to conform within the luggage and not leak onto other contents.
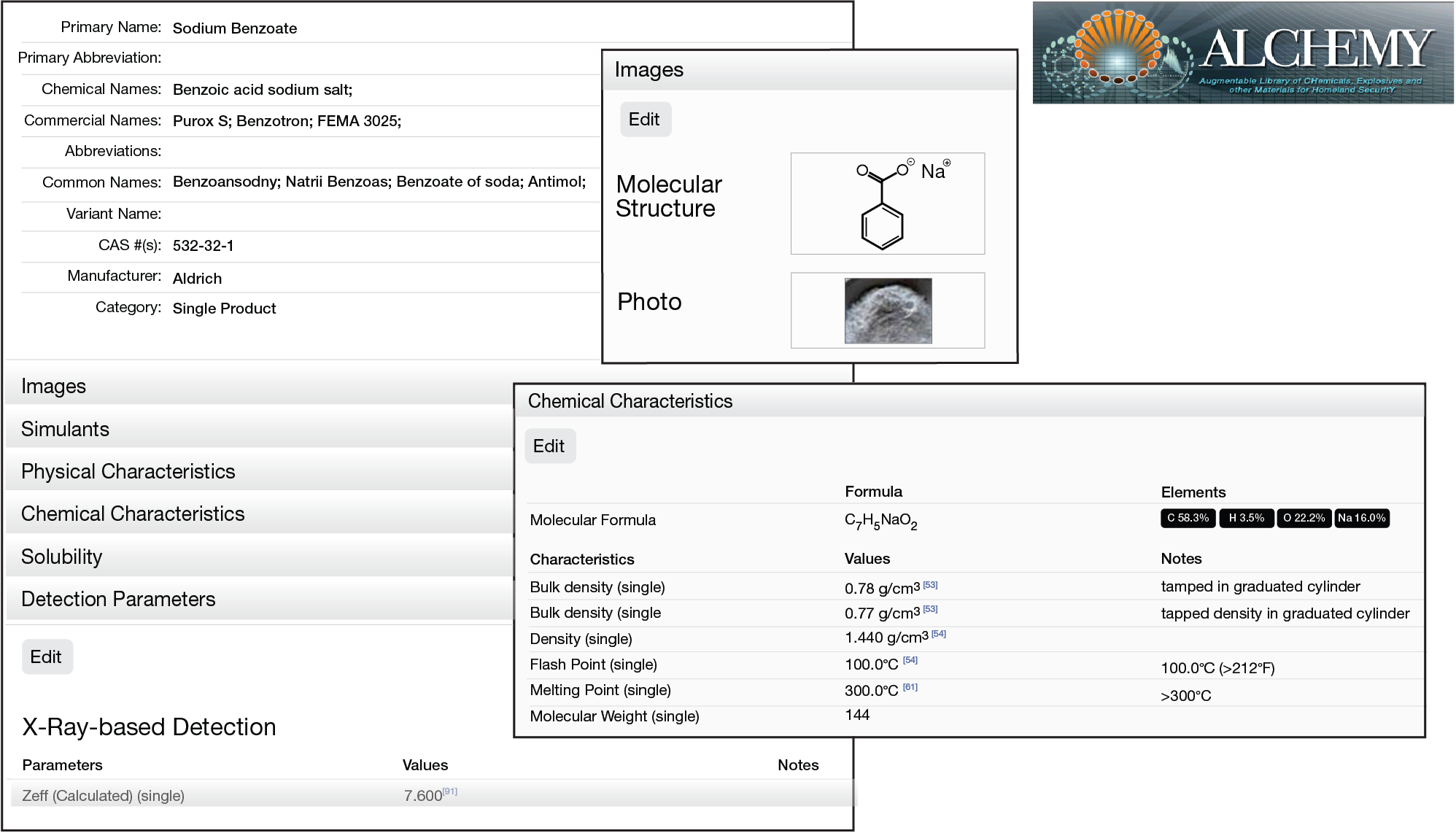
During its 15+ years of technical support to TSA, APL has cataloged physical and chemical properties of hundreds of explosives, precursors, and related materials, as well as simulants (commercial, APL developed, and component materials). These properties include calculated and measured values for various detection technologies, such as x-ray, millimeter wave, optical, and nuclear-based systems (e.g., percentage of nitrogen density). Under tasking from TSA, APL developed a database that serves as a reference guide to enable efficient simulant development. The Augmentable Library of CHemicals, Explosives and other Materials for homeland securitY (ALCHEMY) database contains hundreds of records with referenced values (literature, measurement details, etc.) as well as online tools to aid in simulant design (Figure 2). The team also developed a quality management plan for ALCHEMY measurements that includes standard operating procedures for measuring properties during any simulant development activities.
Figure 2
Example windows from the ALCHEMY database. This APL-developed database contains hundreds of records with referenced values as well as online tools to aid in simulant design.
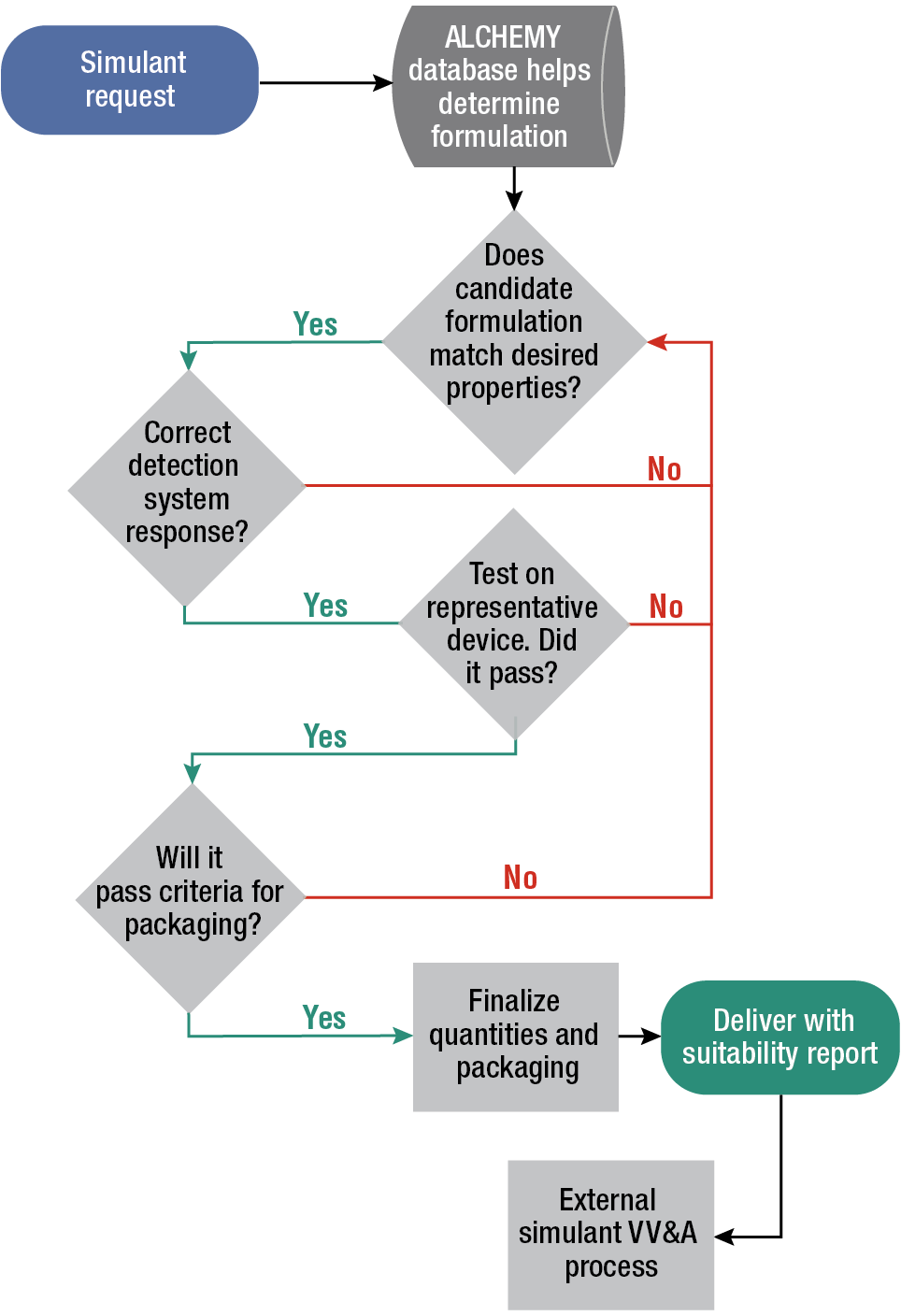
In addition to developing the database and standard operating procedures for property measurement, the team constructed a simulant development process, shown as a flowchart in Figure 3, and an approach to independent verification and validation (IV&V). In general, the design of simulant candidates follows a methodical process, using information from the ALCHEMY database to determine what commercial formulations might exist (and could be leveraged), what previously developed simulant formulations could be used, and what previously characterized materials could be combined.
Figure 3
APL simulant development process. In general, this methodical process leverages an intake form that includes information on the simulant along with data from the ALCHEMY database to determine the formulation. Subsequent steps ensure thorough testing, and the process concludes with delivery of the simulant followed by IV&V.
This process has been implemented many times over the years to provide simulants and test articles to TSA and DHS for a variety of detection technologies. These technologies have included x-ray, millimeter wave, and neutron/gamma-based detection systems for applications in baggage, person-borne, and cargo screening. For the TSA-based simulants, the team created a form to capture all the relevant parties’ requirements and priorities. The form documents basic information on the requested simulant and its application, required detection performance (particularly, the desired fidelity between target energetic and simulant materials), and operating conditions (for example, whether the material will be used indoors or outdoors, details on any interfering equipment, and how the material might be packaged into the test articles). While the specific process described in this article was developed by APL, several other organizations have developed similar approaches. All of the developers recently came together to draft the first-ever ASTM standard for the development of explosive simulants,8 which leverages input from APL.
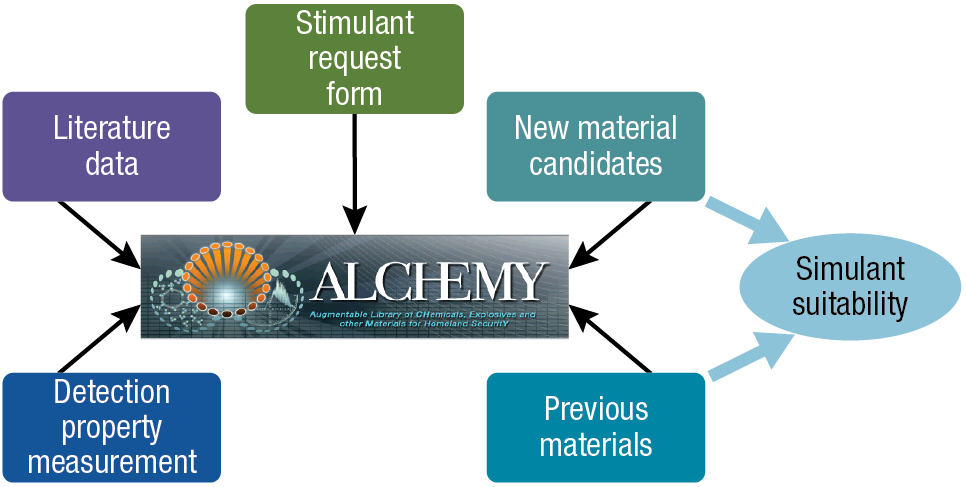
Figure 4 shows another way to visualize the simulant development process, where the ALCHEMY database is used as the central “tool” that takes input from the simulant request form, literature data, and detection property measurement. The ALCHEMY tool can be used to explore previously developed materials and to generate new material candidate mixtures (based on existing components) that can be used to examine simulant suitability in accordance with the desired target–simulant fidelity requirements.
Figure 4
Another visualization of the simulant development process. Here the ALCHEMY database is used as the central tool. It takes input from the simulant request form, literature data, and detection property measurement to provide both previously developed materials and new material candidates.
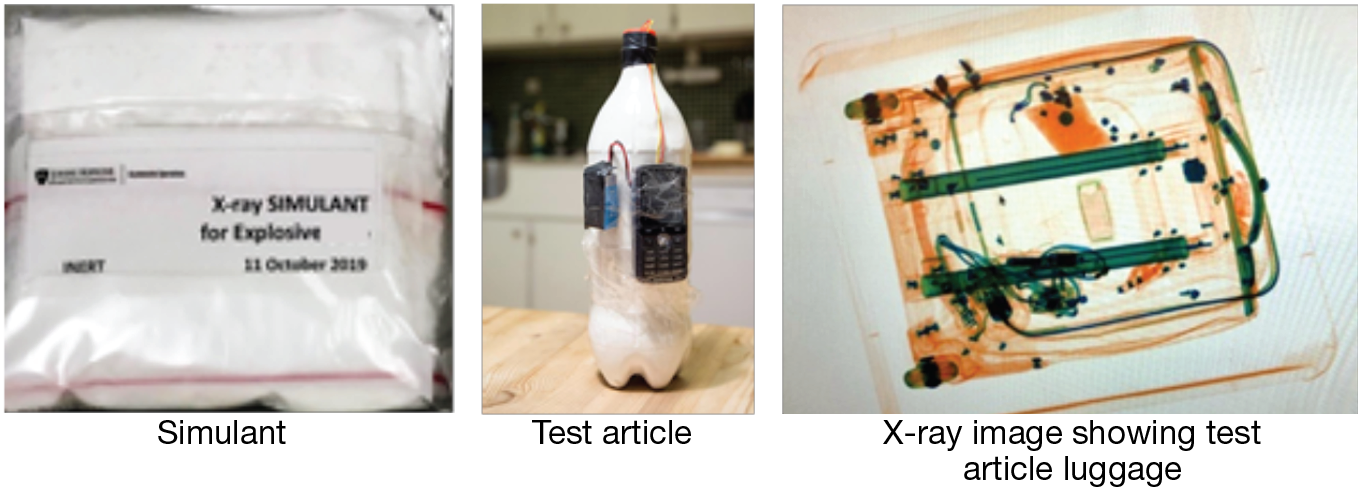
As discussed above and illustrated in Figure 5, a detection-based application of simulant development for energetics includes the formulation of a simulant material, which can then be packaged into a test article (here, an improvised explosive device, or IED) and placed into luggage in order to generate an x-ray image for testing.
Figure 5
Detection-based application. Shown are a packaged simulant, a test article (an IED), and an x-ray image of a test article in luggage.
Chemical Simulants
The recent growth of CWA use described in the introduction highlights the need to develop novel methods for decontamination, destruction, and detection. The Chemical and Biological Weapons Conventions prohibit the widespread use of chemical and biological warfare agents for testing purposes, so simulants must be used. Even if agents were readily available, the needs of development and training programs are best met with simulants. Simulants minimize the risk to personnel and the contamination of equipment and the test location. In addition, working with agents can be cost prohibitive and is limited to certified locations. For these reasons, simulants for chemical and biological warfare agents are crucial for passive defensive research as well as developmental and operational testing. Simply put, simulants allow more testing and training in more settings over a shorter period of time and with fewer restrictions. The simulant data obtained can be used to inform final validation studies with CWAs.
Chemical simulants can facilitate the development of novel mitigation strategies since, by definition, they lack the toxicity of the agent. Chemical simulants may be structurally similar to the agent, may have similar reactivity under a given set of conditions, or both. Similar to the development of explosive simulants described above, the selection of the optimal chemical simulant for a given application combines the iterative activities of literature search, laboratory experimentation, and computational approaches.
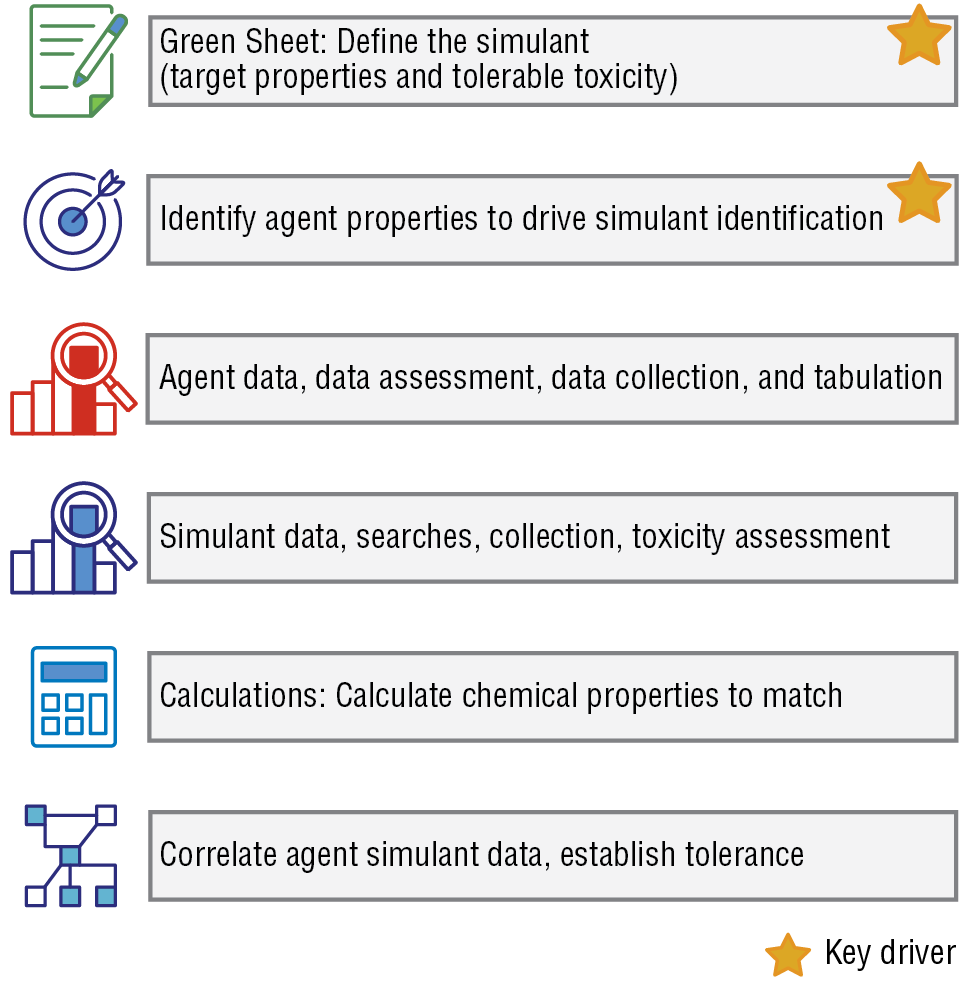
APL has been developing CWA simulants since 2006 and has found that certain tasks are critical to all simulant selection efforts. We have developed a tool set to aid in the simulant selection process. The tools used to down-select from candidate simulants provide a template to guide the chemist and the nonspecialist end user in identifying a CWA simulant that meets the end user’s needs (Figure 6).
Figure 6
The simulant down-selection tool set. These tools guide the identification of a CWA simulant that meets end-user needs.
The simulant down-selection process starts with a series of questions, called the “Green Sheet” (Figure 7), designed to identify the simulant’s intended use. Once the intended use has been determined, the next step is identification of agent properties relevant to that use. These properties, the most important components of the process, are then used to drive the simulant selection process. Selecting the proper simulant relies on the correlation of quality simulant and agent data, regardless of the ultimate use of the simulant. The higher the fidelity of the agent data, the more likely that the selected simulant will mimic the desired target agent behavior. Examples of agent data used in APL’s simulant selections include chemical reactivity with decontamination solutions, physiochemical properties (e.g., solubility, vapor pressure, octanol−water partition coefficient, surface tension, viscosity, and thermal reactivity), and common synthetic pathways. If agent data are unavailable, they must be calculated computationally or collected at a government surety laboratory (a facility where agents are analyzed and other services are performed in a safe and secure environment; surety laboratories are used to analyze chemical, biological, and nuclear materials and to ensure the safety of the materials and the people working with them). These properties are prioritized to help drive the development process. Simulant physiochemical data can be collected from open-source data or computationally determined. Wherever possible, simulants are also evaluated using the same experimental conditions that were used to collect agent data. Finally, the agent and simulant data are correlated. The process is discussed in more detail below.
Figure 7
The Green Sheet. Answering a series of questions that define the end user’s simulant requirements is the start and a key driver of the down-selection process.
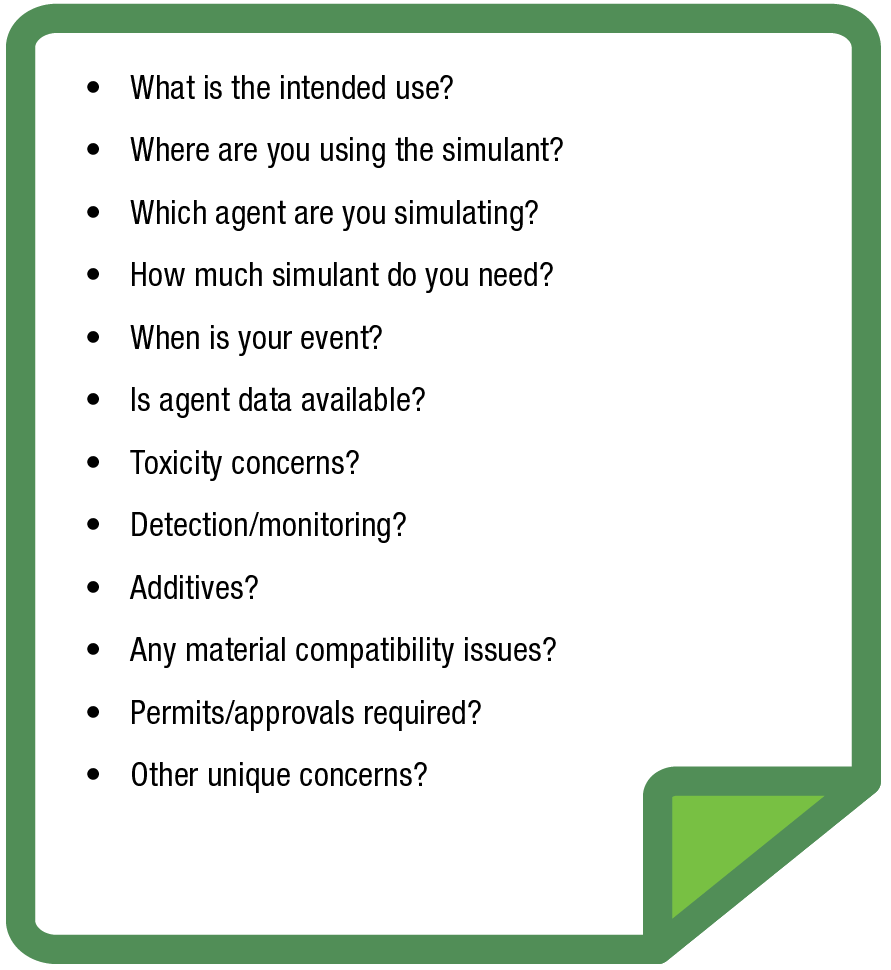
As mentioned, the process begins with the Green Sheet, a series of questions that define the end user’s simulant requirements. These include: What is the intended use? Will the simulant be used in a laboratory with trained chemists, or will it be used outside for fieldwork? If the simulant will be used in the laboratory by trained chemists, smaller quantities of simulant and higher levels of toxicity may be acceptable since engineering controls will be available. On the other hand, if the simulant will be used in outdoor fieldwork, larger quantities of simulant with lower toxicity levels are required, along with site location approvals. Determining how much simulant is needed will help determine whether the simulant can be purchased commercially or will need to be synthesized. Answering this question also helps in establishing timelines since it can take a long time to procure some chemicals.
Based on the end-user requirements defined in the Green Sheet, the agent properties required for the effort can be identified and prioritized. For example, using a simulant in an outdoor test may necessitate library updates to the detection equipment being used in the test. Obtaining the CWA data is the most critical part of the simulant selection process. These studies are expensive and can be performed only at government surety laboratories. If experimental data are unavailable, the physical properties can be modeled computationally.
Once the desired agent properties are selected, the simulant candidates are sought from the open literature. If any of the physical properties of the simulant are unavailable, they can be measured or computationally calculated. The simulant candidates are further down-selected by the criteria set by the Green Sheet. At this point, simulant candidates are narrowed to a short list. Laboratory studies are performed to evaluate simulant performance, ideally using the same procedures used to collect the agent data. For some simulant use cases, end users would like to be able to detect the simulant using their operator tools (specific detection technologies). In these cases, the simulant candidates can be further down-selected to be compatible with the detection technology. A colorimetric or fluorometric indicator is another option that can be added to the simulant formulation and provides a visual indication of simulant location. During the selection process, it is important to ensure that any additive has similar properties to the agent. For example, the additive would have a similar octanol–water partition coefficient to facilitate decontamination studies. Next, it is important to consider any material compatibility issues. For example, simulants used for testing on sensitive equipment should be compatible with the surface materials and should not damage the equipment.
Although each use case is unique and can have requirements that are not captured by the initial question list, a completed Green Sheet provides a template to identify the key agent properties that drive the simulant selection. The agent properties that must be retained in the simulant for a specific application vary considerably with the simulant use. Finally, the selected simulant formulation is evaluated under the experimental conditions used above and the results are correlated to the agent. This will show how the simulant compares to the agent. For example, compared with the agent, a decontamination simulant could correlate as x times easier or harder to remove from a given surface when following the use-specific instructions.
This is best illustrated with an example.
Chemical Simulant Example
The Defense Threat Reduction Agency (DTRA) Automated, Detailed Equipment Decontamination for Land Vehicles (Auto-Decon) advanced technology demonstration program required a chemical weapons simulant for use in large-scale outdoor training of the United States Army Chemical Corps. The simulant was used for a baseline detailed equipment decontamination and training of the 71st Chemical Company (Figure 8).
Figure 8
Photo from a decontamination training exercise. APL supported this outdoor field test evaluating decontamination of military vehicles. (Used with permission from Esry.10)

APL worked with the Auto-Decon team to define and prioritize the target properties of the simulant. The Auto-Decon Green Sheet is shown in Figure 9. With the readily available agent data, as well as the protocol used for decontamination with hot, soapy water (HSW),9 the APL team developed a list of simulant candidates.
The initial simulant list included more than 100 candidates selected primarily from commercial off-the-shelf flavor and fragrance compounds. The simulant properties identified on the Green Sheet were molecular weight, melting point, boiling point, vapor pressure, octanol–water partition coefficient, and toxicity. The octanol–water coefficient is a first-principle descriptor of how a compound will interact in a mixed aqueous/organic system (like soapy water). For reference, compounds with a low octanol–water coefficient are more water soluble than organic soluble. Any molecule with an octanol–water coefficient between that for VX and HD was selected for further evaluation.
Figure 9
The Green Sheet for DTRA’s Auto-Decon training event. The sheet identifies simulant use and target properties. With this information, along with agent data and the protocol used for decontamination, the APL team developed a list of simulant candidates.

The Green Sheet indicated that the final simulant formulation must contain a fluorophore visible to the naked eye. The down-selection process is shown in Figure 10. The APL team initially selected candidate fluorophores on the basis of the criteria that they were expected to be colorless in solution under ambient light and to fluoresce under long-wave ultraviolet (UV) light irradiation. The fluorophores were required to be fluorescent when exposed to the long-wave UV light. The initial evaluation of the fluorophores involved dissolving each molecule in ethanol at a concentration of 50 mM (Figure 11). Fluorophores that gave brightly colored solutions and fluorophores that did not dissolve in ethanol were eliminated from consideration.
Figure 10
The Auto-Decon simulant down-selection process. The APL team initially selected candidate fluorophores that were expected to be colorless in solution under ambient light and to fluoresce blue under ultraviolet (UV) light irradiation.
Figure 11
Representative simulant with an added fluorophore on a CARC coupon. This photograph was taken outside in direct sunlight. The blue fluorescence is readily visible from the handheld UV light source.

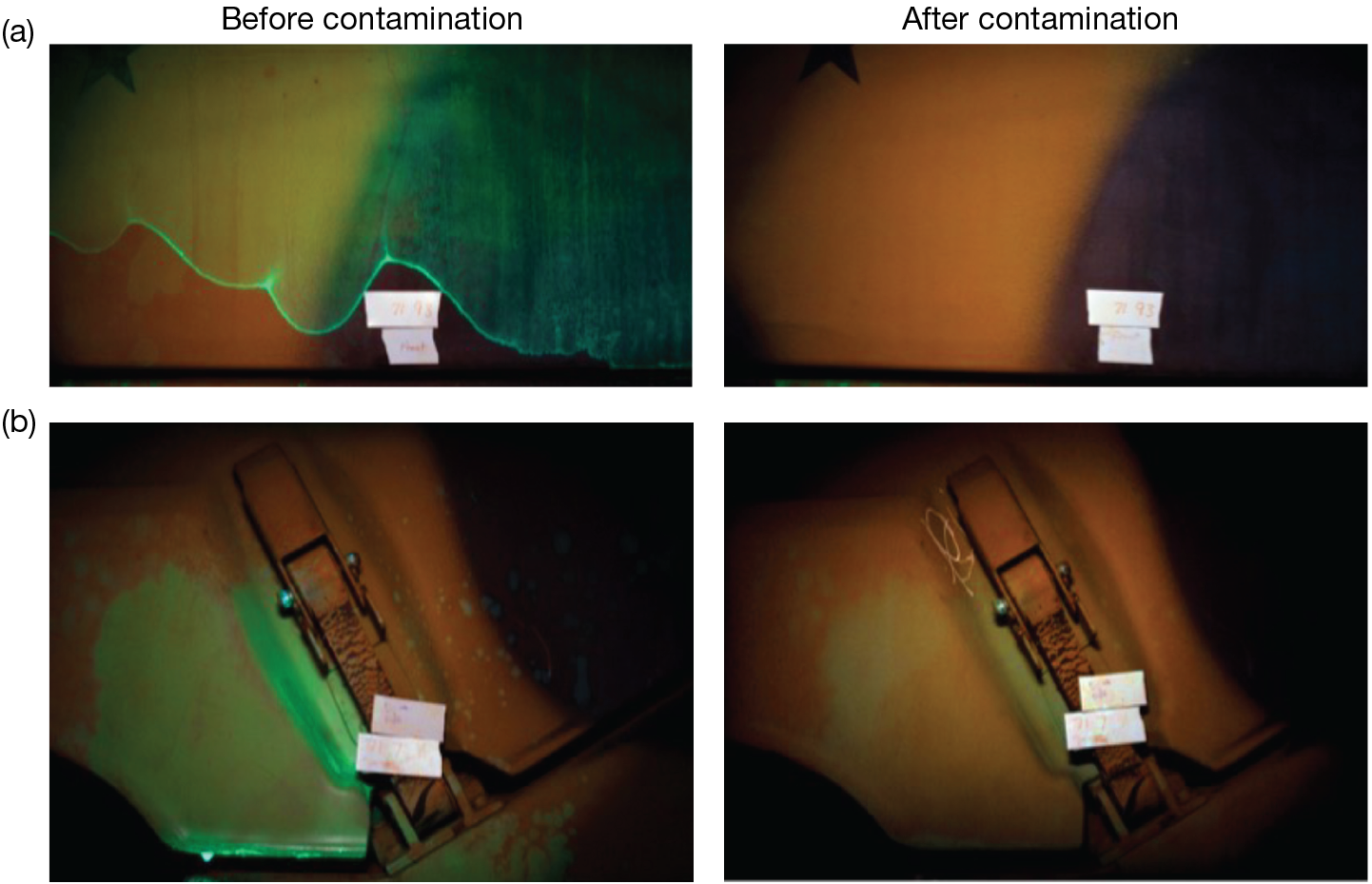
The APL team then evaluated the remaining simulant candidates’ solubilities of the fluorophores. They evaluated various simulant–fluorophore pairs for their visibility under UV light on chemical-agent-resistant coating (CARC)-painted stainless steel coupons. Simulant–fluorophore pairs that were not readily visible on CARC were eliminated. Since the fluorophore serves as a marker for the simulant formulation, it is important that it behave like the simulant under HSW decontamination. The octanol–water coefficients were calculated for selected fluorophores using commercially available software. Finally, selected simulant–fluorophore pairs were evaluated in decontamination studies using the same protocols used to collect agent data. Figure 12 shows results from the Auto-Decon test. The top photos (a) show before decontamination and after successful decontamination of a part of a military vehicle. The bottom photos (b) show a failed decontamination. The vehicle shown in the bottom photos was returned to the decontamination line for additional cleaning. The ability to quickly access decontamination efficiency in the field provided real-time feedback to end users so they could evaluate performance and protocols.
Figure 12
Photos from the Auto-Decon field test. Left, simulant formulation applied before decontamination. Right, after decontamination. (a) Successful decontamination; (b) unsuccessful decontamination. (Used with permission from Esry.10)
The Auto-Decon simulant use case had some unique requirements. Since the simulant was applied to military vehicles, it was important that it did not damage the vehicles. In addition to performing decontamination evaluation studies on CARC coupons, the APL team also tested with glass, cast acrylic, and extruded acrylic to determine whether these surfaces would be compatible with the simulant and the decontaminant. Additionally, because the field test’s decontamination wash solutions were released into the environment, the simulant formulation had to be biodegradable to comply with requirements of the state where the simulant was used in training. These requirements represent the unique needs for this use case and demonstrate the need to account for simulant use during early stages of field test planning to ensure there is sufficient time to resolve any site-specific issues.
Conclusion
The hazardous nature of explosives and CWAs has driven the development of simulants, which mimic specific properties and characteristics of the materials of interest. The use of simulants facilitates development of tools and tactics to protect the warfighter and other frontline security professionals. Using a systems engineering approach, APL has created a simulant development process that includes a methodology for the design and selection of scenario-specific simulants. This process provides simulant materials that are ideally suited to a specific detection or other application, as defined by the mission. Our selection process has been embraced by the user community and has been leveraged for numerous use cases. Because of the extreme safety risk and cost of studies using energetic materials and CWAs, simulant efforts will continue to provide valuable, impactful information to our sponsors.
- START (National Consortium for the Study of Terrorism and Responses to Terrorism), Global Terrorism Database 1970–2020 [data file], 2022, https://www.start.umd.edu/gtd/.
- J. A. Vale, T. C. Marrs, and R. L. Maynard, “Novichok: A murderous nerve agent attack in the UK,” Clin. Tox., vol. 56, no. 11, pp. 1093–1097, 2018, https://doi.org/10.1080/15563650.2018.1469759.
- P. R. Chai, E. W. Boyer, H. Al-Nahhas, and T. B. Erickson, “Toxic chemical weapons of assassination and warfare: Nerve agents VX and sarin,” Tox. Comm., vol. 1, no. 1, pp. 21–23, 2017, https://doi.org/10.1080/24734306.2017.1373503.
- R. Pita and J. Domingo, “The use of chemical weapons in the Syrian conflict,” Toxics, vol. 2, no. 3, pp. 391–402, 2014, https://doi.org/10.3390/toxics2030391.
- J. Patočka, “Syria conflict and chemical weapons: What is the reality?,” Mil. Med. Sci. Lett., vol. 85, no. 1, pp. 39–43, 2016, https://doi.org/10.31482/mmsl.2016.006.
- “2022 United States Bomb Data Center (USBDC) Explosives Incident Report (EIR),” DHS TRIPwire, Jun. 2, 2023.
- “Annex on chemicals,” in the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, Jan. 13, 1993, https://www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals.
- “Standard Guide for Development, Verification, Validation, and Documentation of Explosive and Contraband Simulants for Security Screening Systems,” draft standard submitted to the ASTM International E54 Committee on Homeland Security Applications, Nov. 2023.
- Z. Zander, M. Waller, M. Shue, M. Sheahy, M. Hall, and P. Humphreys, “Evaluation of candidate decontaminants for automated decontamination program,” Edgewood Chemical Biological Center Tech. Rep. no. 673, Apr. 2009.
- D. Esry, “Automated Detailed Equipment Decontamination (Auto Decon) Advanced Technology Demonstration (ATD) Baseline Demonstration Simulant Photo Report,” KCP-613-8786, Honeywell, FM&T, Kansas City, MO, Aug. 2010.
David S. Lawrence is a project leader and manager in APL’s Asymmetric Operations Sector. He earned a B.S. in chemistry from Carnegie Mellon University and a Ph.D. in organic chemistry from the University of Rochester. David leads various programs involving detection instrumentation for chemical warfare agents and explosives analytical chemistry, as well as air and water sample preparation and analysis. He has more than 23 years of experience supporting homeland protection programs, including chemical, biological, radiological, nuclear, and explosives (CBRNE) sensor design and development, market surveys, evaluation, and deployment, as well as environmental analyses. He also has 20 years of experience in organic chemistry and biochemistry, specializing in the synthesis of complex organic and organometallic molecules, with research interests in spectroscopic analysis and detection.
Kelly A. Van Houten is the supervisor of APL’s Applied Chemistry and Physics Group, which is responsible for developing solutions to detect, defeat, and deny chemical and nuclear threats. She has a B.A. in chemistry from Johns Hopkins University, a Ph.D. in chemistry from the University of Maryland, College Park, and an MBA from Johns Hopkins University. She has over 20 years of experience developing novel detection methodologies for organophosphates, designing and synthesizing chemical simulants, and developing chemistry-based warfighter tools.