Press Release
Johns Hopkins APL Team Makes Powerful Advances on a Better Thermal Battery
Researchers at the Johns Hopkins Applied Physics Laboratory (APL) in Laurel, Maryland, have developed a new thermal battery for national security applications. The battery utilizes a nickel-aluminum heat source and offers several performance enhancements, including improved energy density, increased operating times and decreased operating temperatures and size.
Thermal batteries were originally developed by the Germans during World War II as a power source for V2 rockets. Shortly thereafter, the United States adapted them as power sources for missiles and proximity fuzes because of their reliability, long shelf life and power density.
As missile systems became faster and more complex over the last several decades, the Navy’s Program Executive Office for Integrated Warfare Systems (IWS) was interested in developing a battery to support them. As the technical direction agent for Standard Missile programs, responsible for performing critical experiments and developing prototypes that address missile limitations and advance capabilities of Navy systems, APL answered that call.
The team’s goal was clear: to build a thermal battery with a smaller footprint and higher power density. After promising early results, APL engineers expanded and fine-tuned their work with support from the Navy’s Surface Ship Weapons program and the Office of Naval Research.
“To make a smaller but more powerful battery, we had to change the electrolyte and cathode materials within it, and advancements in cathode chemistries haven’t changed for several decades,” explained Yo-Rhin Rhim, a materials scientist in APL’s Air and Missile Defense Sector (AMDS) and the principal investigator on the battery project. “To change those chemistries, we had to take a step back and adjust the heat source.”
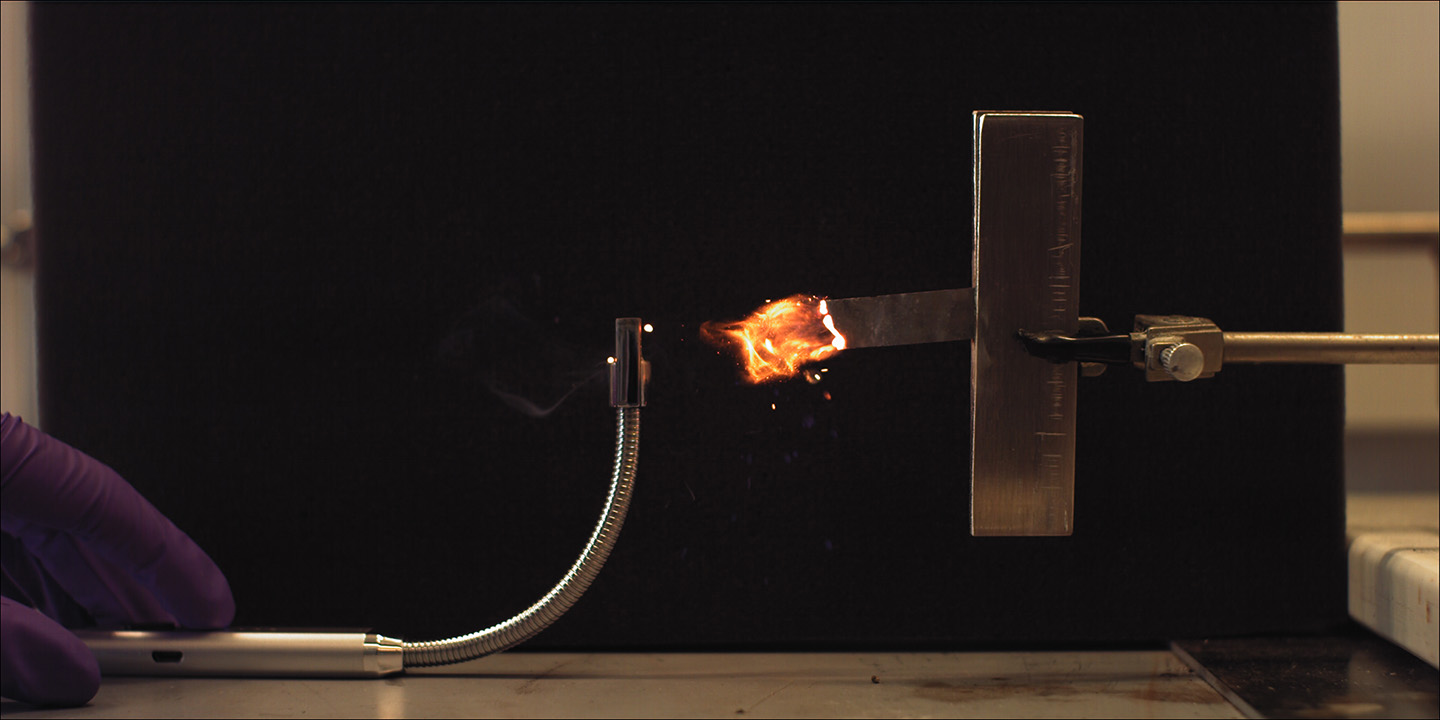
Anodes, cathodes and electrolytes are the three basic building blocks for batteries. In thermal batteries, there’s a fourth component — a heat source, or heat pellets. Most heat pellets used in thermal batteries are made of an iron metal powder combined with potassium perchlorate. While iron/potassium perchlorate pellets are extremely dependable, they don’t cooperate well with varying chemistries the team was analyzing for new cathodes and electrolytes.
“To use power from a battery in your phone or in your car, we rely on the electrolyte to shuttle ions from the anode — the negative side of the battery, into the cathode — the positive side. The electrolytes in those batteries are typically salty liquid solutions,” said David Burns, an energetic-materials chemist in AMDS. “In thermal batteries, the electrolyte is essentially a solid salt, which allows it to be stable and inactive for long-term storage. Once the heat source ignites to melt that salt, the battery suddenly becomes electrochemically active.”
In APL’s thermal battery, an electric igniter activates nickel-aluminum heat pellets, setting off a chain reaction that melts the electrolytes, activates the rest of the battery and powers the system it is attached to. The team’s developments resulted in a battery that is roughly 60% smaller than most thermal batteries used today.
“When we were looking for cheaper heat source solutions, we were thinking, ‘I wish we could get aluminum foil, dip it in nickel and just see if it works,’” said Rhim. “And that’s what we did. We went to the store and got several types of aluminum foil, and then our team members — David Lee and Nick Nowicki — electrolessly deposited nickel onto it and lit it with a match.”
The team began refining those parameters and perfecting the chemistries.
“One of the biggest challenges we faced was making sure that once the battery was ignited, it fired fully and the materials within became molten and were properly contained so they wouldn’t leak and short the battery,” said Alex Yuan, a materials engineer in APL’s Research and Exploratory Development Department. “To overcome these challenges, [team member] John King led development of an energetic mesh capable of both capturing the molten material and releasing additional heat, upon battery ignition, to enhance propagation of the pellet burning front.”
To build a prototype and test these capabilities, APL partnered with Sandia National Laboratories, a leading thermal battery manufacturer for Department of Defense applications. In February, the battery comprising a suite of APL proprietary technologies completed several successful rounds of testing.
“This success has been many years in the making and wouldn’t have happened without knowledge and collaboration across the Laboratory,” said Rhim.