Now You See It, Now You Don't
Taking advantage of these seasons, the team examined the molecular data on short- and long-time periods just before the comet’s southern hemisphere entered summer and then again just as its summer ended. As reported in their study, published March 10 in Nature Astronomy, the team found that as the southern hemisphere turned away and was sufficiently far from the Sun, the link between oxygen and water disappeared. The amount of water coming off the comet dropped precipitously, so instead the oxygen seemed strongly linked to carbon dioxide and carbon monoxide, which the comet was still emitting.
“There’s no way that should be possible under the previous explanations suggested,” Luspay-Kuti said. “If oxygen were primordial and tied to water in its formation, there shouldn’t be any time that oxygen strongly correlates with carbon monoxide and carbon dioxide but not water.”
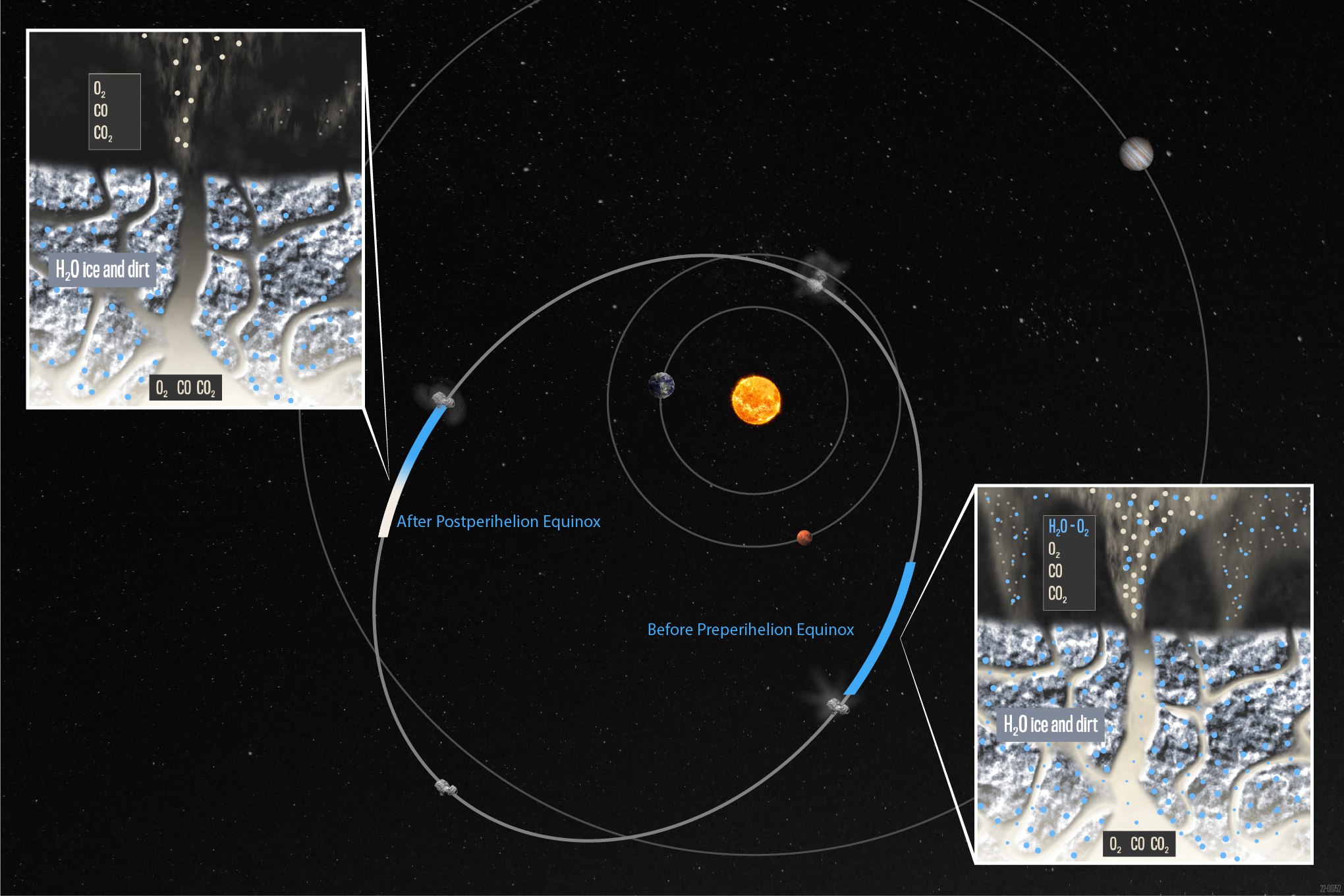
The team instead proposed the comet’s oxygen doesn’t come from water but from two reservoirs: one made of oxygen, carbon monoxide and carbon dioxide deep inside the comet’s rocky nucleus, and a shallower pocket closer to the surface where oxygen chemically combines with water ice molecules.
The idea goes like this: A deep reservoir of oxygen, carbon monoxide and carbon dioxide ice is constantly emitting gases because oxygen, carbon dioxide and carbon monoxide all vaporize at very low temperatures. As oxygen traverses from the comet’s interior toward the surface, however, some chemically inserts into water ice (a major constituent of the comet’s nucleus) to form a second, shallower oxygen reservoir. But water ice vaporizes at a much higher temperature than oxygen, so until the Sun sufficiently heats the surface and vaporizes the water ice, the oxygen is stuck.
The consequence is that oxygen can accumulate in this shallow reservoir for long periods until the comet surface is finally warmed enough for water ice to vaporize, releasing a plume far richer in oxygen than was actually present in the comet.
“Put another way, the oxygen abundances measured in the comet’s coma aren’t necessarily reflecting its abundances in the comet’s nucleus,” Luspay-Kuti explained.
The comet would consequently also vacillate with the seasons between strongly associating with water (when the Sun heats the surface) and strongly associating with carbon dioxide and carbon monoxide (when that surface faces away from the Sun and the comet is sufficiently far) — exactly what Rosetta observed.
“This isn’t just one explanation: It’s the explanation because there is no other possibility,” said Olivier Mousis, a planetary scientist from France’s Aix-Marseille Université and a study co-author. “If oxygen were just coming from the surface, you wouldn’t see these trends observed by Rosetta.”
The major implication, he said, is that it means comet 67P’s oxygen is, in fact, oxygen that accreted at the beginning of the solar system. It’s just that it’s only a fraction of what people had thought.
Luspay-Kuti said she wants to probe the topic more deeply by examining the comet’s minor molecular species, such as methane and ethane, and their correlation with molecular oxygen and other major species. She suspects this will help researchers get a better idea of the type of ice that the oxygen was incorporated into.
“You still have to come up with a way to incorporate the oxygen into the comet,” Luspay-Kuti said, considering that the amount of oxygen is still higher than seen in most molecular clouds. But she said she expected a majority of researchers will welcome the study and its conclusions with a sigh of relief.