Press Release
Johns Hopkins APL, Medicine Researchers Apply Data Fusion to Improve Breast Cancer Detection
Breast cancer is the most common cancer among women worldwide, according to the World Health Organization, claiming hundreds of thousands of lives each year. Detecting the disease early has been shown to dramatically decrease its morbidity and mortality, but there are limitations to current screening methods.
Researchers at the Johns Hopkins Applied Physics Laboratory (APL) in Laurel, Maryland, are collaborating with radiologists from Johns Hopkins Medicine (JHM) to develop an upstream data fusion (UDF) method — leveraging artificial intelligence — that promises to significantly improve the accuracy of breast cancer screening. The work, which is funded through an ASPIRE Award from The Mark Foundation for Cancer Research, was presented at the 15th International Workshop on Breast Imaging (IWBI) online program and published in the event proceedings.
APL’s David Porter — an expert in UDF — is the lead author of the paper, and Dr. Lisa Mullen, of JHM, is the principal investigator of the project. The research team also includes Michael Williams, a mathematician in APL’s Force Projection Sector; William Walton, an electrical engineer in APL’s Asymmetric Operations Sector; Dr. Susan Harvey (formerly of JHM); and Keith Peyton, a defense consultant.
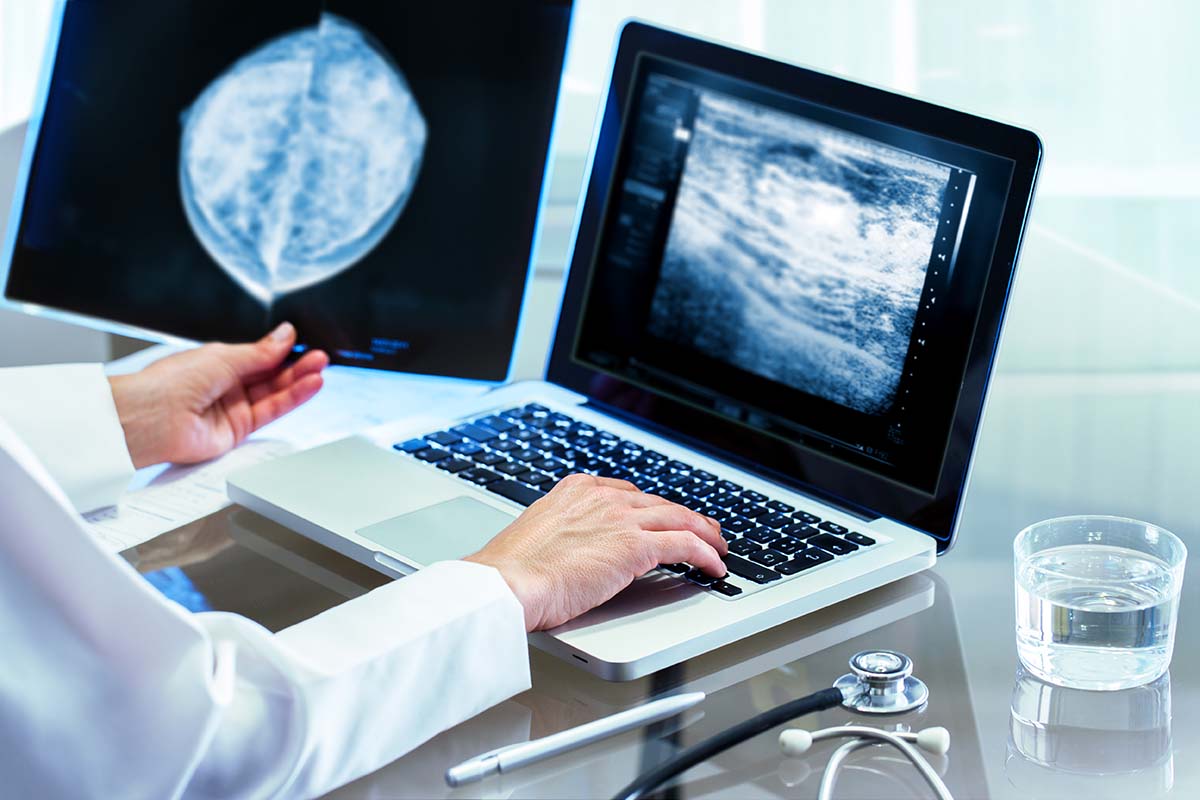
Annual mammography screening has been shown to decrease breast cancer mortality rates by as much as 38%. There are two widely used examinations: full field digital mammography (FFDM) and digital breast tomosynthesis (DBT), also known as 3D mammography. FFDM consists of two images of each breast. With DBT, multiple projections of each breast are obtained and then reconstructed into 1-millimeter slices.
A major, general limitation of mammography is the overlap of normal breast tissue, which obscures lesions. But the 1-millimeter slices generated by DBT decrease the superimposition of tissue, thereby improving the detection of lesions and increasing the accuracy of the diagnosis.
Despite DBT’s benefits and performance, the IWBI paper indicates that challenges and limitations still exist. For one, breast imaging radiologists can be overwhelmed by the sheer volume of studies associated with widespread screening programs, a problem made worse by DBT, which produces 200 to 400 images per study compared to only four images per FFDM study. Additionally, the task of interpreting mammography is difficult, with inter- and intra-reader variability as high as 30%.
The authors point out that both screening methods have been known to generate false positives — with associated costs of up to $4 billion annually in the United States — and are limited in their ability to clearly detect invasive cancers in dense breast tissue. Even when the screening is supplemented with ultrasound, false-positive diagnoses still occur.
The paper presents an innovative approach to this critical problem, leveraging UDF to combine the strengths of these complementary breast imaging modalities. UDF refers to the processing, exploitation and fusion of sensor data as close to the raw sensor data feed as possible. APL has been pioneering UDF applications since the early 2000s.
“We originally developed the UDF concept to aid military operators who were faced with a situation that they described as ‘drowning in data but starving for information,’” explained Porter, a co-inventor of two patented data fusion modeling systems for other applications. “For breast cancer detection and diagnosis, radiologists face a similar challenge as they work surrounded by an array of computer screens, reviewing hundreds or even thousands of images for each patient.”
Using data from DBT and breast ultrasound studies, the team trained, validated and tested an existing AI method, including UDF, for the detection of breast cancer. The UDF and machine-learning methodology produced fused detections using lesion features and locations, as well as association (or matching) of lesions from DBT and ultrasound. It then calculated a probability of malignancy, location certainty and probability of correct data association.
“Upstream data fusion showed success in our prior work for reducing false alarms in other applications, and it is starting to show promise for breast cancer also,” Porter said. According to Mullen, “This technology could help reduce radiologist interpretation time, while also improving cancer detection and reducing false positives.”
The team envisions maturing and transitioning the UDF and machine-learning technologies into standard clinical practices in highly developed as well as low-resourced areas, in the United States and globally.